Hepatopulmonary syndrome in patients with porto-sinusoidal vascular disorder: Characteristics and outcome
- PMID: 40171298
- PMCID: PMC11960633
- DOI: 10.1016/j.jhepr.2024.101310
Hepatopulmonary syndrome in patients with porto-sinusoidal vascular disorder: Characteristics and outcome
Abstract
Background & aims: Porto-sinusoidal vascular disorder (PSVD) is a rare cause of portal hypertension. Data on hepatopulmonary syndrome (HPS) in PSVD are limited. This study aimed to determine the associated factors, plasma mediators, and evolution of HPS in patients with PSVD.
Methods: Multicenter observational study of patients with PSVD with signs of portal hypertension in whom contrast-enhanced transthoracic echocardiography (CE-TTE) was performed.
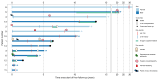
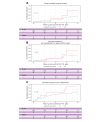
Results: Among 196 patients with PSVD who underwent CE-TTE in 17 centers, 14 (7% [95% confidence interval 3-11%]) had a confirmed diagnosis of HPS. Patients with HPS more frequently had a genetic disorder associated with PSVD (50% vs. 6%, p <0.001), especially telomere biology disorders (p <0.001). Liver function was less preserved in patients with HPS, because they had lower prothrombin index (63% vs. 86%, p = 0.04), higher serum total bilirubin (37 μmol/L vs. 14 μmol/L, p <0.001), and lower serum albumin (32 g/L vs. 38 g/L, p <0.001). HPS tended to be associated with more portal venule obliterations (p = 0.085) and with nodular liver architecture (p = 0.069). Plasma concentrations of Angiopoietin-2, ICAM3, and Tie2 were higher in patients with HPS (p = 0.02, p = 0.04, p = 0.01, respectively). Out of the 14 patients with HPS, five underwent liver transplantation after a median follow-up of 34 months. Overall cumulative incidence of liver-related events and of death was similar between patients with and without HPS, when considering liver transplantation for HPS as a competing risk.
Conclusions: HPS in patients with PSVD was associated with genetic disorders, less preserved liver function, and higher plasma concentrations of angiogenic mediators. When applying HPS model for end-stage liver disease exception policy for liver transplantation, overall survival of patients with PSVD and HPS was similar to that of patients with PSVD without HPS.
Impact and implications: Hepatopulmonary syndrome (HPS) is a rare complication of porto-sinusoidal vascular disorder (PSVD). This multicentric study found that patients with PSVD and HPS had less preserved liver function, and harbored genetic disorders more frequently (especially telomere biology disorders) than patients without HPS. HPS did not negatively impact transplantation-free survival when applying HPS MELD exception policy for liver transplantation through a competitive risk analysis. Our findings suggest that patients with PSVD with respiratory symptoms and/or telomere biology disorders may benefit from systematic screening for HPS.
Keywords: Hepatopulmonary syndrome; Hypoxemia; Liver; Lung–liver interaction; Portal hypertension; Vascular liver disease.
© 2024 The Author(s).
Conflict of interest statement
P-ER has received research funding from Terrafirma and acted as consultant for Hemostod, Mursla, Genfit, Boehringer Ingelheim, and Abbelight, and received speaker fees from Tillots pharma and AbbVie. CC received research funding from Gilead and Ipsen, and speaker fees from AbbVie, Intercept, and Gilead. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- De Gottardi A., Rautou P.E., Schouten J., et al. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4:399–411. - PubMed
-
- Schouten J.N.L., Garcia-Pagan J.C., Valla D.C., et al. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54:1071–1081. - PubMed
-
- Khanna R., Sarin S.K. Non-cirrhotic portal hypertension – diagnosis and management. J Hepatol. 2014;60:421–441. - PubMed
-
- Schouten J.N.L., Nevens F., Hansen B., et al. Idiopathic noncirrhotic portal hypertension is associated with poor survival: results of a long-term cohort study. Aliment Pharmacol Ther. 2012;35:1424–1433. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

