Effective gene therapy for metachromatic leukodystrophy achieved with minimal lentiviral genomic integrations
- PMID: 40171445
- PMCID: PMC11960508
- DOI: 10.1016/j.omtn.2025.102464
Effective gene therapy for metachromatic leukodystrophy achieved with minimal lentiviral genomic integrations
Abstract
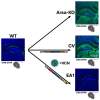
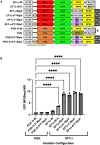
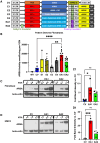
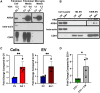
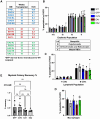
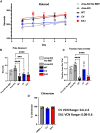
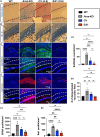
Metachromatic leukodystrophy (MLD) is a fatal lysosomal storage disease characterized by the deficient enzymatic activity of arylsulfatase A (ARSA). Combined autologous hematopoietic stem cell transplantion (HSCT) with lentiviral (LV)-based gene therapy has great potential to treat MLD. Achieving the optimal balance between high enzyme production for therapeutic efficacy and maintaining a low vector copy number (VCN) is crucial. Insufficient enzyme levels can lead to the progression of motor symptoms, undermining treatment goals. Conversely, elevated VCN increases the risk of genotoxicity, which poses safety concerns, and contributes to higher production costs, making the therapy less accessible. Striking this balance is essential to maximize clinical benefit while minimizing risks and costs. To address this need, we increased the expression of ARSA cDNA at single integration by generating novel LVs, optimizing ARSA expression and enhancing safety. In addition, our vectors achieved optimal transduction in mouse and human hematopoietic stem cells (HSCs) with minimal multiplicity of infection (MOI). Our top-performing vector (EA1) showed at least 4× more ARSA activity than the currently US and European Union (EU)-approved vector and a superior ability to secrete vesicle-associated ARSA, a critical modality to transfer functional enzymes from microglia to oligodendrocytes. Three-month-old Arsa-knockout (KO) MLD mice transplanted with Arsa-KO bone marrow (BM) cells transduced with 0.6 VCN of EA1 demonstrated behavior and CNS histology matching wild-type (WT) mice. Our novel vector boosts efficacy while improving safety as a robust approach for treating MLD patients.
Keywords: MLD; MT: Delivery Strategies; gene complementation; gene therapy; lysosomal storage disorder; metachromatic leukodystrophy; novel lentiviral vector; toxicity; translational research.
© 2025 The Authors.
Conflict of interest statement
S.R. is a scientific advisory board member of Ionis Pharmaceuticals, Vifor, and Disc Medicine. Present to last 5 years: S.R. has been or is a consultant for GSK, BMS, Incyte, Cambridge Healthcare Res, Celgene Corporation, Catenion, First Manhattan Co., FORMA Therapeutics, Ghost Tree Capital, Keros Therapeutics, Noble insight, Protagonist Therapeutics, Sanofi Aventis U.S., Slingshot Insight, Spexis AG, Techspert.io, BVF Partners L.P., Rallybio LLC, venBio Select LLC, ExpertConnect LLC, and LifeSci Capital. The vector EA1 is protected in the patent-CHOP 2022-023: “Novel lentiviral vectors for the treatment of Multiple Sulfatase Deficiency (MSD), Metachromatic Leukodystrophy (MLD) and related disorder of sulfatase deficiency” (CHOP, L.T. and S.R.).
Figures

Update of
-
Effective Gene Therapy for Metachromatic Leukodystrophy Achieved with Minimal Lentiviral Genomic Integrations.bioRxiv [Preprint]. 2024 Mar 14:2024.03.14.584404. doi: 10.1101/2024.03.14.584404. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2025 Jan 25;36(1):102464. doi: 10.1016/j.omtn.2025.102464. PMID: 38559013 Free PMC article. Updated. Preprint.
References
-
- Schmidt J.L., Pizzino A., Nicholl J., Foley A., Wang Y., Rosenfeld J.A., Mighion L., Bean L., da Silva C., Cho M.T., et al. Estimating the relative frequency of leukodystrophies and recommendations for carrier screening in the era of next-generation sequencing. Am. J. Med. Genet. 2020;182:1906–1912. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

