Endothelial insulin-like growth factor-1 signalling regulates vascular barrier function and atherogenesis
- PMID: 40171617
- PMCID: PMC12236071
- DOI: 10.1093/cvr/cvaf055
Endothelial insulin-like growth factor-1 signalling regulates vascular barrier function and atherogenesis
Abstract
Aims: Progressive deposition of cholesterol in the arterial wall characterizes atherosclerosis, which underpins most cases of myocardial infarction and stroke. Insulin-like growth factor-1 (IGF-1) is a hormone that regulates systemic growth and metabolism and possesses anti-atherosclerotic properties. We asked whether endothelial-restricted augmentation of IGF-1 signalling is sufficient to suppress atherogenesis.
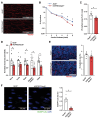
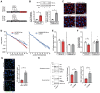
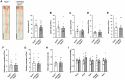
Methods and results: We generated mice with endothelial-restricted over-expression of human wild-type (WT) IGF-1R (hIGFREO/ApoE-/-) or a signalling-defective K1003R mutant human IGF-1R (mIGFREO/ApoE-/-) and compared them with their respective ApoE-/- littermates. hIGFREO/ApoE-/- had less atherosclerosis, circulating leucocytes, arterial cholesterol uptake, and vascular leakage in multiple organs, whereas mIGFREO/ApoE-/- did not exhibit these phenomena. Over-expressing WT IGF-1R in human umbilical vein endothelial cells (HUVECs) altered the localization of tight junction proteins and reduced paracellular leakage across their monolayers, whilst over-expression of K1003R IGF-1R did not have these effects. Moreover, only over-expression of WT IGF-1R reduced HUVEC internalization of cholesterol-rich low-density lipoprotein particles and increased their association of these particles with clathrin, but not caveolin-1, implicating it in vesicular uptake of lipoproteins. Endothelial over-expression of WT vs. K1003R IGF-1R also reduced expression of YAP/TAZ target genes and nuclear localization of TAZ, which may be relevant to its impact on vascular barrier and atherogenesis.
Conclusion: Endothelial IGF-1 signalling modulates both para- and transcellular vascular barrier function. Beyond reducing atherosclerosis, this could have relevance to many diseases associated with abnormal vascular permeability.
Keywords: Atherosclerosis; Endothelial; Insulin-like growth factor-1 receptor; Vascular permeability.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.M.C. has received speaker’s fees from Janssen Oncology for work unrelated to this project.
Figures

Comment in
-
Endothelial IGF-1 signalling: a conductor of vascular barrier function and low-density lipoprotein trafficking in atherogenesis.Cardiovasc Res. 2025 Jul 8;121(7):983-984. doi: 10.1093/cvr/cvaf082. Cardiovasc Res. 2025. PMID: 40359234 No abstract available.
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, Vaccaro Kd, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Leo DD, Degenhardt L, Delossantos A, Denenberg J, Jarlais DD, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J-P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, León FD, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P-H, Yip P, Zabetian A, Zheng Z-J, Lopez AD, Murray CJL. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–2128. - PMC - PubMed
-
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diab Res Clin Pract 2013;103:137–149. - PubMed
-
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126. - PubMed
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

