Delivery of Human iPSC-Derived RPE Cells in Healthy Minipig Retina Results in Interaction Between Photoreceptors and Transplanted Cells
- PMID: 40171949
- PMCID: PMC12120741
- DOI: 10.1002/advs.202412301
Delivery of Human iPSC-Derived RPE Cells in Healthy Minipig Retina Results in Interaction Between Photoreceptors and Transplanted Cells
Abstract
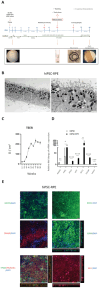
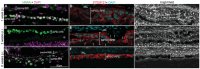
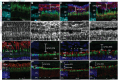
In late stages of inherited and acquired retinal diseases such as Stargardt disease (STGD) or dry age-related macular degeneration (AMD), loss of retinal pigment epithelia (RPE) cells and subsequently photoreceptors in the macular area result in a dramatic decline of central visual function. Repopulating this area with functional RPE cells may prevent or decline the progression of photoreceptor loss. In the present study, the viability, survival, and integration of human induced pluripotent stem cell (hiPSC)-derived RPE cells (hiPSC-RPE) is assessed generated using clinical-grade protocol and cultured on a clinically relevant scaffold (poly-L-lactide-co-D, L-lactide, PDLLA) after subretinal implantation in immunosuppressed minipigs for up to 6 weeks. It is shown that transplanted hiPSC-RPE cells maintain the RPE cell features such as cell polarity, hexagonal shape, and cell-cell contacts, and interact closely with photoreceptor outer segments without signs of gliosis or neuroinflammation throughout the entire period of examination. In addition, an efficient immunosuppressing strategy with a continuous supply of tacrolimus is applied. Continuous verification and improvement of existing protocols are crucial for its translation to the clinic. The results support the use of hiPSC-RPE on PDLLA scaffold as a cell replacement therapeutic approach for RPE degenerative diseases.
Keywords: Human induced pluripotent stem cells;minipigs; age‐related macular degeneration; cell therapy; retina; retinal degeneration; retinal pigment epithelium.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Huang D., Heath Jeffery R. C., Aung‐Htut M. T., McLenachan S., Fletcher S., Wilton S. D., Chen F. K., Ophthalmic Genet. 2022, 43, 1. - PubMed
MeSH terms
Grants and funding
- TO01000107/Norway Grants and Technology Agency of the Czech Republic
- CZ.02.01.01/00/22_008/0004562/European Regional Development Fund by Programme Johannes Amos Comenius
- 21180/Foundation AFM-Telethon
- 202010-10/Fundació la Marató de TV3
- Instituto de Salud Carlos III (ISCIII) grant PI20/01119 co-funded by European Regional Development Fund (FEDER)
LinkOut - more resources
Full Text Sources
