Inhalable Polymeric Nanoparticle Containing Triphenylphosphanium Bromide-modified Sonosensitizer for Enhanced Therapy of Acute Bacterial Pneumonia
- PMID: 40171966
- PMCID: PMC12165071
- DOI: 10.1002/advs.202417469
Inhalable Polymeric Nanoparticle Containing Triphenylphosphanium Bromide-modified Sonosensitizer for Enhanced Therapy of Acute Bacterial Pneumonia
Abstract
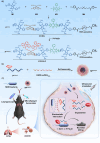
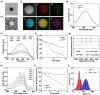
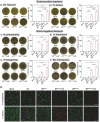
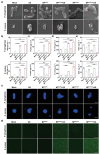
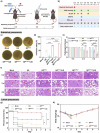
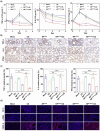
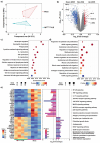
Sonodynamic therapy (SDT) has good feasibility to deeply seated infections, but SDT alone is insufficient being highly effective against multidrug-resistant (MDR) bacteria. SDT combined with triphenylphosphanium bromide (P+Ph3Br-) is expected to solve this problem. This work develops a pseudo-conjugated polymer PFCPS-P containing cationic P+Ph3Br--modified sonosensitizer FCPS (FCPS-P) and ROS-sensitive thioketal bonds. PFCPS-P is assembled with DSPE-mPEG2000 to generate nanoparticle NPFCPS-P. FCPS has SDT effect and generates ROS under ultrasound (US) stimulation. ROS triggers the degradation of NPFCPS-P and release of FCPS-P, endowing highly favored biosafety. FCPS-P targets to bacterial surface through electrostatic interaction and achieves bacterial killing under a synergistic action of SDT and P+Ph3Br-. In vitro, NPFCPS-P+US gives >90% inhibition rates against MDR ESKAPE pathogens, moreover, it causes bacterial metabolic disorders including inhibited nucleic acid synthesis, disordered energy metabolism, excessive oxidative stress, and suppressed biofilm formation and virulence. In mice, NPFCPS-P+US exhibits a 99.3% bactericidal rate in Pseudomonas aeruginosa-induced sublethal pneumonia and renders a 90% animal survival rate in lethal pneumonia, and additionally immunological staining and transcriptomics analyses reveal that NPFCPS-P+US induces inhibited inflammatory response and accelerated lung injury repair. Taken together, NPFCPS-P+US is a promising antibiotics-alternative strategy for treating deeply seated bacterial infections.
Keywords: ESKAPE pathogens; bacterial pneumonia; cationic triphenylphosphanium bromide; pseudo‐conjugated polymer; sonodynamic therapy.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Treatment of Acute Wound Infections by Degradable Polymer Nanoparticle with a Synergistic Photothermal and Chemodynamic Strategy.Adv Sci (Weinh). 2024 May;11(17):e2309624. doi: 10.1002/advs.202309624. Epub 2024 Feb 26. Adv Sci (Weinh). 2024. PMID: 38408124 Free PMC article.
-
An In vitro Study on the Antibacterial Effect of a Combined Photodynamic and Sonodynamic Therapy Using IR780 Iodide-loaded Mesoporous Silica Nanoparticles Against P. aeruginosa and Multi-Drug Resistant P. aeruginosa.Recent Adv Antiinfect Drug Discov. 2025;20(1):64-76. doi: 10.2174/0127724344309438240529064221. Recent Adv Antiinfect Drug Discov. 2025. PMID: 40302549
-
Degradable antimicrobial polycarbonates with unexpected activity and selectivity for treating multidrug-resistant Klebsiella pneumoniae lung infection in mice.Acta Biomater. 2019 Aug;94:268-280. doi: 10.1016/j.actbio.2019.05.057. Epub 2019 May 24. Acta Biomater. 2019. PMID: 31129359
-
Sonodynamic therapy-based nanoplatforms for combating bacterial infections.Ultrason Sonochem. 2023 Nov;100:106617. doi: 10.1016/j.ultsonch.2023.106617. Epub 2023 Sep 23. Ultrason Sonochem. 2023. PMID: 37769588 Free PMC article. Review.
-
Sonodynamic and Acoustically Responsive Nanodrug Delivery System: Cancer Application.Int J Nanomedicine. 2024 Nov 11;19:11767-11788. doi: 10.2147/IJN.S496028. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39553460 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
