Papaverine Targets STAT Signaling: A Dual-Action Therapy Option Against SARS-CoV-2
- PMID: 40171981
- PMCID: PMC11963225
- DOI: 10.1002/jmv.70319
Papaverine Targets STAT Signaling: A Dual-Action Therapy Option Against SARS-CoV-2
Abstract
Papaverine (PV) has been previously identified as a promising candidate in SARS-CoV-2 repurposing screens. In this study, we further investigated both its antiviral and immunomodulatory properties. PV displayed antiviral efficacy against SARS-CoV-2 and influenza A viruses H1N1 and H5N1 in single infection as well as in co-infection. We demonstrated PV's activity against various SARS-CoV-2 variants and identified its action at the post-entry stage of the viral life cycle. Notably, treatment of air-liquid interface (ALI) cultures of primary bronchial epithelial cells with PV significantly inhibited SARS-CoV-2 levels. Additionally, PV was found to attenuate interferon (IFN) signaling independently of viral infection. Mechanistically, PV decreased the activation of the IFN-stimulated response element following stimulation with all three IFN types by suppressing STAT1 and STAT2 phosphorylation and nuclear translocation. Furthermore, the combination of PV with approved COVID-19 therapeutics molnupiravir and remdesivir demonstrated synergistic effects. Given its immunomodulatory effects and clinical availability, PV shows promising potential as a component for combination therapy against COVID-19.
Keywords: SARS‐CoV‐2; antiviral; immunomodulatory; interferon; papaverine.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
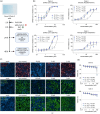
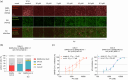

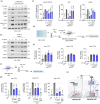
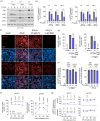
References
-
- Mathieu E., Ritchie H., Rodés‐Guirao L., et al., Coronavirus Pandemic (COVID‐19) (2020), accessed October 6, 2023, https://ourworldindata.org/coronavirus.
-
- Wang L., Berger N. A., Davis P. B., Kaelber D. C., Volkow N. D., and Xu R., COVID‐19 Rebound After Paxlovid and Molnupiravir During January‐June 2022 (Infectious Diseases (except HIV/AIDS), 2022), 10.1101/2022.06.21.22276724. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

