Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction
- PMID: 40172303
- PMCID: PMC11963282
- DOI: 10.1002/alz.70062
Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction
Erratum in
-
Correction to "Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction".Alzheimers Dement. 2025 Aug;21(8):e70576. doi: 10.1002/alz.70576. Alzheimers Dement. 2025. PMID: 40775664 Free PMC article. No abstract available.
Abstract
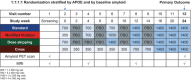
Introduction: TRAILBLAZER-ALZ 6 (NCT05738486) is a multicenter, double-blind, ongoing phase 3b study in early symptomatic Alzheimer's disease.
Methods: Participants (n = 843) were randomized 1:1:1:1 (standard + three alternative donanemab dosing arms). Primary outcome was relative risk reduction (RRR) of amyloid-related imaging abnormalities with edema/effusions (ARIA-E) at 24 weeks assessed with Bayesian logistic regression. Amyloid plaque levels by positron emission tomography and serum donanemab pharmacokinetics were measured.
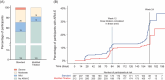
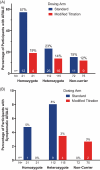
Results: ARIA-E frequencies for standard, modified titration, dose skipping, and Cmax arms were 23.7%, 13.7%, 18.6%, and 18.3%, respectively, at 24 weeks and similar at 52 weeks: 24.2%, 15.6%, 18.6%, and 18.8%, respectively. Modified titration met the 24-week primary outcome with 94% probability of achieving ≥ 20% RRR versus the standard arm. Modified titration also had significantly lower ARIA-E severity, but similar cumulative exposure and mean amyloid reduction compared to the standard arm.
Discussion: Gradual up-titration of dose significantly reduced ARIA-E risk while demonstrating comparable pharmacokinetics/pharmacodynamics compared to standard dosing.
Highlights: In TRAILBLAZER-ALZ 6, the amyloid-related imaging abnormalities-edema/effusions (ARIA-E) frequency was 13.7% in the modified titration arm compared to 23.7% in the standard arm at week 24. The modified titration arm met the primary endpoint of ARIA-E relative risk reduction at 24 weeks versus the standard arm. The modified titration versus standard arm at week 24 had comparable non-ARIA-E related safety profile, amyloid reduction, plasma phosphorylated tau217 reduction, cumulative exposure, and pharmacokinetics. Data at week 52 were consistent with week 24 results.
Keywords: Alzheimer's disease; amyloid; amyloid‐related imaging abnormalities; donanemab; phosphorylated tau217; titration.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
HW, ESMN, PA, RK, DOS, IG, SS‐Eli Lilly and Company, SWA, PMH, SEE, DAB, ECC, MAM, and JRS are employees and minor shareholders of Eli Lilly and Company. NCF reports consulting fees from Biogen, Eisai, Eli Lilly, and Roche (all paid to UCL); he has received fees for serving on a data safety monitoring board for Biogen and on an advisory board for Abbvie; acknowledges grant support from the Alzheimer's Society (UK), Alzheimer's Research UK, Rosetrees Trust, the Sigrid Rausing Trust, and the UK NIHR UCLH Biomedical Research Centre. SMG reports receiving consulting fees from Eli Lilly and Company and participates in safety data monitoring boards or advisory boards for IQVIA/Washington University. SS‐Butler Hospital receives research support for conducting clinical trials through grants or contracts from Biogen, Eisai, Genentech, Roche, CognitionRx, Lilly, and Janssen; he has received consulting fees from Abbvie, Acumen, Alector, Biogen, Biohaven, Cognition, Eisai, Fujirebio, Genentech, Kisbee, Laqbcorp, Lilly, Merck, Neurophet, NovoNordisk, Prothena, Quest, and Roche. Author disclosures are available in the supporting information.
Figures

References
-
- Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023;19(2):658‐670. - PubMed
-
- Kisunla (donaneamb‐azbt) [product insert]. 2024. Eli Lilly and Company.
-
- Leqembi (lecanemab‐irmb) [package insert]. 2023. Eisai Inc.
-
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691‐1704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

