The probiotic Lacticaseibacillus rhamnosus GG supplementation reduces Salmonella load and modulates growth, intestinal morphology, gut microbiota, and immune responses in chickens
- PMID: 40172512
- PMCID: PMC12070740
- DOI: 10.1128/iai.00420-24
The probiotic Lacticaseibacillus rhamnosus GG supplementation reduces Salmonella load and modulates growth, intestinal morphology, gut microbiota, and immune responses in chickens
Abstract
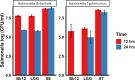
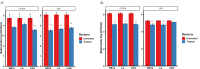
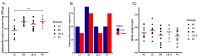
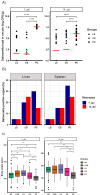
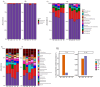
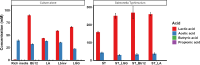
Salmonella, a leading cause of foodborne illnesses, is primarily transmitted to humans through the consumption of contaminated poultry products. The increasing resistance of Salmonella to antibiotics and lack of cross-protection by vaccines necessitate new control strategies in poultry production systems. This study assessed the efficacy of probiotics against Salmonella Typhimurium (ST) and Salmonella Enteritidis (SE). Lactobacillus acidophilus (LA), Lacticaseibacillus rhamnosus GG (LGG), and Bifidobacterium animalis subsp. lactis (Bb12) showed inhibition of ST and SE in agar well diffusion assay, with stable inhibitory properties. In co-culture assay, both LGG and Bb12 completely suppressed ST and SE growth. Liquid chromatography-with tandem mass spectrometry (LC-MS/MS) analysis of the LGG and Bb12 cell-free culture supernatant identified novel bioactive peptides with anti-Salmonella properties. Administering LGG in drinking water of chickens raised on built-up litter floor in experimental conditions significantly reduced the ST load (5.95 logs and 3.74 on 7 days post-infection [dpi] and 14 dpi, respectively). Gut microbiota analysis revealed increased abundance of several beneficial genera such as Butyricicoccus, Erysipelatoclostridium, Flavonifractor, and Bacillus in LGG-treated groups. Histomorphometry analysis demonstrated increased villus height (VH) and VH by crypt depth ratio in the ileum of the LGG-treated group on 14 dpi. These results highlight LGG as a promising probiotic for controlling Salmonella in chickens and reducing transmission to humans. The beneficial properties of LGG are attributed to the production of antimicrobial peptides, microbiota modulation, and enhanced intestinal integrity.IMPORTANCESalmonella is the leading cause of foodborne illnesses in the United States and worldwide. It is primarily transmitted through contaminated poultry and poultry products (eggs and poultry meat). Increasing resistance of Salmonella to antibiotics and lack of cross-protection by vaccines necessitate new control strategies to reduce Salmonella in poultry production system and minimize human infections. Probiotics, which are live beneficial microorganisms when administered in an optimum amount, have been increasingly used in recent years as alternatives to antibiotics to promote health. Our study showed that LGG exhibited superior probiotics properties and significantly reduced Salmonella load in chickens. Thus, LGG supplementation is a promising approach to prevent Salmonella infection and enhance performance of poultry thereby enhance food safety, proper antibiotic stewardship and public health.
Keywords: LGG; Salmonella; chickens; foodborne pathogens; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




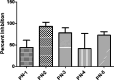
References
-
- Get the facts about Salmonella. 2024. Available from: https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-a.... Retrieved 13 Dec 2024.
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

