Monkeypox virus H3L protein as the target antigen for developing neutralizing antibody and serological assay
- PMID: 40172630
- PMCID: PMC11965195
- DOI: 10.1007/s00253-025-13466-6
Monkeypox virus H3L protein as the target antigen for developing neutralizing antibody and serological assay
Abstract
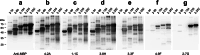
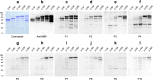
The large number of atypical monkeypox (Mpox) cases caused by emerging monkeypox virus (MPXV) strains was recently found in countries and regions where the Mpox was not reported before. Diagnostic tools and therapeutic agents are important countermeasures for preventing Mpox outbreak. H3L protein is the important surface antigen of MPXV for binding to host cell receptors and mediating viral infection. A broad range of murine anti-MPXV H3L monoclonal antibodies (mAbs) recognizing various binding epitopes have been generated in the study. The rapid test composed of the mAbs 4-2A and 3-3F can specifically detect H3L protein and MPXV virion. The mAb 3-3F exhibited strong MPXV neutralizing activity in a complement-dependent manner. Notably, 3-3F binds to a unique epitope within residues 35-89 of H3L protein. The serum samples collected from Mpox patients barely bound to the N-terminal portion of H3L protein ranging from 2 to 89 residues, indicating that the content of the 3-3F-like antibody is very low in Mpox patient sera. In contrast, the seropositivity was mostly observed using the C-terminal portion of H3L protein ranging from 185 to 282 residues as the target antigen in the immunoblot analysis. Taken together, the anti-MPXV H3L mAb can be developed as the Mpox diagnostic and therapeutic agents. Furthermore, H3L protein is the promising biomarker for serological analysis. KEY POINTS: •Anti-H3L mAbs can cross-react with H3L proteins in MPXV and VACV virions. •The LFIA rapid test using the mAbs 4-2A and 3-3F can specifically detect MPXV. •MPXV was neutralized by mAb 3-3F in a complement-dependent manner.
Keywords: Complement; H3L protein; Lateral flow immunochromatographic assay; Monkeypox virus (MPXV); Neutralizing antibody; Serological assay.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical statement: The animals’ care and protocol have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University (approval number: NTU-112-EL-00080). A written informed consent was obtained from all individual Mpox patients in the study, which was approved by the Institutional Review Board of National Taiwan University Hospital. Conflict of interest: The authors declare no competing interest.
Figures





References
-
- Benhnia MR, Maybeno M, Blum D, Aguilar-Sino R, Matho M, Meng X, Head S, Felgner PL, Zajonc DM, Koriazova L, Kato S, Burton DR, Xiang Y, Crowe JE Jr, Peters B, Crotty S (2013) Unusual features of vaccinia virus extracellular virion form neutralization resistance revealed in human antibody responses to the smallpox vaccine. J Virol 87(3):1569–1585 - PMC - PubMed
-
- Cheng YC, Chang SC (2021) Development and biochemical characterization of the monoclonal antibodies for specific detection of the emerging H5N8 and H5Nx avian influenza virus hemagglutinins. Appl Microbiol Biotechnol 105(1):235–245 - PubMed
-
- Cho W, Park S, Kim HJ, Lee M, Choi YS, Yeo SG, Lee J, Koyanagi A, Jacob L, Smith L, Rahmati M, Ahmad S, Fond G, Boyer L, Rhee SY, Lee SW, Shin JI, Woo HG, Yon DK (2024) Clinical characteristics and outcomes of patients with mpox during the 2022 mpox outbreak compared with those before the outbreak: a systematic review and meta-analysis. Rev Med Virol 34(1):e2508 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

