Chronotherapy With Once-Daily Osilodrostat Improves Cortisol Rhythm, Quality of Life, and Sleep in Cushing's Syndrome
- PMID: 40172910
- PMCID: PMC12623021
- DOI: 10.1210/clinem/dgaf206
Chronotherapy With Once-Daily Osilodrostat Improves Cortisol Rhythm, Quality of Life, and Sleep in Cushing's Syndrome
Abstract
Context: Medical therapy for Cushing syndrome (CS) typically aims to reduce daily cortisol output without addressing circadian rhythm restoration. No licensed drugs target this goal.
Objective: We investigated the efficacy and safety of timed, once-daily osilodrostat administration in improving circadian cortisol profiles in CS.
Methods: A prospective, multicenter study evaluated patients with well-controlled CS on a stable twice-daily osilodrostat therapy before and 60 to 90 days after transitioning to a single equivalent daily dose at 19:00 ± 1 hour. Circadian steroid analysis was performed on saliva, serum, and urine using ultra-high performance liquid chromatography-tandem mass spectrometry. Additional assessments included cardio-metabolic markers, quality of life, sleep function, and safety outcomes.
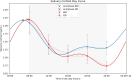
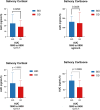
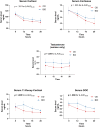
Results: Sixteen patients (4 males; 7 pituitary, mean age 53.3 ± 11.8 years) were enrolled. At baseline, CS was well-controlled with a mean osilodrostat dose of 4.2 ± 1.3 mg. After transitioning, salivary cortisol exposure decreased significantly during the afternoon to early morning period (AUC16:00-08:00: -6.1 [-0.15 to -12.1] ng/mL/h, P = .029). Quality of life and sleep improved (CushingQoL: +4.2, P = .029; Pittsburgh Sleep Quality Index: -1.7, P = .049). Serum steroid precursors, including 11-deoxycorticosterone (-3.1 ng/mL/h, P = .008) and 11-deoxycortisol (-17.8 ng/mL/h, P = .005), decreased. Eight patients advancing dosing to 16:00 ± 1 hour showed comparable reductions, with phase shifts in acrophase and nadir. No patients developed adrenal insufficiency, liver toxicity, electrocardiogram abnormalities, or loss of disease control.
Conclusion: Once-daily osilodrostat effectively and safely treats patients with biochemically controlled CS, improving circadian cortisol profiles, quality of life, and sleep. Findings support further exploration of chronotherapy-based approaches in CS management.
Keywords: Cushing's Disease; Cushing's syndrome; chrono-pharmacology; cortisol circadian rhythm; cortisone; hypercortisolism; medical therapy; saliva; salivary cortisol.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Comment in
-
Soothing a Ruffled Mind: Chronotherapy of Cushing Syndrome.J Clin Endocrinol Metab. 2025 Jul 18:dgaf353. doi: 10.1210/clinem/dgaf353. Online ahead of print. J Clin Endocrinol Metab. 2025. PMID: 40679927 No abstract available.
References
-
- Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611‐629. - PubMed
-
- Gadelha M, Gatto F, Wildemberg LE, Fleseriu M. Cushing's syndrome. Lancet. 2023;402(10418):2237‐2252. - PubMed
-
- Reincke M, Fleseriu M. Cushing syndrome: a review. JAMA. 2023;330(2):170‐181. - PubMed
-
- Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: a review. JAMA. 2023;329(16):1386‐1398. - PubMed
-
- Bertagna X. MANAGEMENT OF ENDOCRINE DISEASE: can we cure Cushing's disease? A personal view. Eur J Endocrinol. 2018;178(5):R183‐R200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

