Knockout of cyclin-dependent kinases 8 and 19 leads to depletion of cyclin C and suppresses spermatogenesis and male fertility in mice
- PMID: 40172945
- PMCID: PMC11964450
- DOI: 10.7554/eLife.96465
Knockout of cyclin-dependent kinases 8 and 19 leads to depletion of cyclin C and suppresses spermatogenesis and male fertility in mice
Abstract
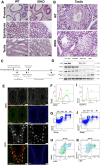
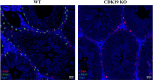
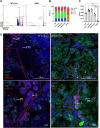
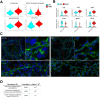
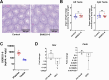
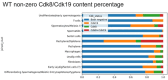
CDK8 and CDK19 paralogs are regulatory kinases associated with the transcriptional Mediator complex. We have generated mice with the systemic inducible Cdk8 knockout on the background of Cdk19 constitutive knockout. Cdk8/19 double knockout (iDKO) males, but not single Cdk8 or Cdk19 KO, had an atrophic reproductive system and were infertile. The iDKO males lacked postmeiotic spermatids and spermatocytes after meiosis I pachytene. Testosterone levels were decreased whereas the amounts of the luteinizing hormone were unchanged. Single-cell RNA sequencing showed marked differences in the expression of steroidogenic genes (such as Cyp17a1, Star, and Fads) in Leydig cells concomitant with alterations in Sertoli cells and spermatocytes, and were likely associated with an impaired synthesis of steroids. Star and Fads were also downregulated in cultured Leydig cells after iDKO. The treatment of primary Leydig cell culture with a CDK8/19 inhibitor did not induce the same changes in gene expression as iDKO, and a prolonged treatment of mice with a CDK8/19 inhibitor did not affect the size of testes. iDKO, in contrast to the single knockouts or treatment with a CDK8/19 kinase inhibitor, led to depletion of cyclin C (CCNC), the binding partner of CDK8/19 that has been implicated in CDK8/19-independent functions. This suggests that the observed phenotype was likely mediated through kinase-independent activities of CDK8/19, such as CCNC stabilization.
Keywords: CDK19; CDK8; Spermatogenesis; developmental biology; mouse.
© 2024, Bruter, Varlamova et al.
Conflict of interest statement
AB, EV, NS, ZA, VM, AT, DK, AK, MU, VB, IB, AN, EA, DM, JL, GS, AF, AS, VM, YS, VT No competing interests declared, MC Director of Research of Senex Biotechnology, Inc, IR Founder and President of SenexBiotechnology, Inc
Figures







Update of
- doi: 10.1101/2023.11.04.564913
- doi: 10.7554/eLife.96465.1
- doi: 10.7554/eLife.96465.2
- doi: 10.7554/eLife.96465.3
References
-
- Aherrahrou R, Kulle AE, Alenina N, Werner R, Vens-Cappell S, Bader M, Schunkert H, Erdmann J, Aherrahrou Z. CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development. Scientific Reports. 2020;10:8792. doi: 10.1038/s41598-020-65601-0. - DOI - PMC - PubMed
-
- Alarcón C, Zaromytidou A-I, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massagué J. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. - DOI - PMC - PubMed
-
- Andolfi C, Bartolini C, Morales E, Gündoğdu B, Puhr M, Guzman J, Wach S, Taubert H, Aigner A, Eder IE, Handle F, Culig Z. MED12 and CDK8/19 modulate androgen receptor activity and enzalutamide response in prostate cancer. Endocrinology. 2024;165:bqae114. doi: 10.1210/endocr/bqae114. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

