Trends in Treatment of Severe Haemophilia and Impact on Inhibitor Assessment by the EUHASS Registry
- PMID: 40173026
- PMCID: PMC12175111
- DOI: 10.1111/hae.70039
Trends in Treatment of Severe Haemophilia and Impact on Inhibitor Assessment by the EUHASS Registry
Abstract
Background: The last 15 years have seen new extended half-life (EHL) recombinant FVIII/IX concentrates and nonreplacement therapy for haemophilia A (emicizumab) introduced in Europe. These changes affect FVIII/IX exposure in previously untreated patients (PUPs) and previously treated patients (PTPs) with severe haemophilia A and B (SHA and SHB) and may modify inhibitor development and/or detection.
Aim: To report trends in treatment for severe haemophilia and concomitant changes in inhibitor incidence.
Methods: Between 2008 and 2022, 97 centres reported inhibitor development against FVIII/IX concentrates to the European Haemophilia Safety Surveillance System (EUHASS). Inhibitors were reported quarterly, and PUPs without inhibitor development annually. Cumulative inhibitor incidences (95% confidence intervals [CI]) were calculated for PUPs and incidence rates/1000 years (CI) for PTPs.
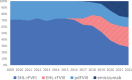
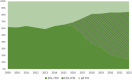
Results: By 2022, SHA-PUPs (n = 1574) received emicizumab (44%), SHL-rFVIII (21.5%), pdFVIII (17.5%) and EHL-rFVIII (17%). SHB-PUPs (n = 236) received EHL-rFIX (79%) and SHL-rFIX (21%). SHA-PTPs (68,772 years) received EHL-rFVIII (31%), SHL-rFVIII (28%), emicizumab (25%), and pdFVIII (15%). SHB PTPs (11,185 years) received EHL-rFIX (69%), pdFIX (15%) and SHL-rFIX (15%). Observed Inhibitor incidence in SHA-PUPs decreased from 24% before 2016 to 6% in 2022 (p < 0.001), and potentially in SHB-PUPs too (from 9% to 3%; p = 0.066), but remained stable in SHA/SHB PTPs.
Conclusion: In 2022, 44% of SHA-PUPs and 25% of SHA-PTPs received emicizumab prophylaxis. Concomitantly, observed inhibitor incidence reduced to 6% in SHA-PUPs. In SHB, EHL-rFIX treatment increased to 79% in SHB-PUPs and 69% in SHB-PTPs. Assessing inhibitor incidence for new concentrates is likely to be hampered by novel treatments causing delayed exposure to FVIII/FIX.
Keywords: PTP; PUP; antibodies; emicizumab; factor VIII; haemophilia; inhibitor; neutralising.
© 2025 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
K.F. has acted as a consultant and participated in expert groups for Bayer, Biogen, CSL Behring, NovoNordisk, and SOBI, has received research grants from Bayer, NovoNordisk, Pfizer, and has given invited educational lectures for Bayer, NovoNordisk, and Pfizer, and has received travel support from Sobi and Bayer. R.L. received honoraria for advisory board participation for CSL Behring, NovoNordisk, Pfizer, Sanquin and Sobi. F.P. participated in the advisory board of CSL Behring, Biomarin, Roche, Sobi, Sanofi and has given invited educational lectures and symposia for Takeda and Spark. A.G. reported no competing interests. R.H. is CEO at MDSAS. T.L. received honoraria for consultancy, advisory board participation and/or invited educational lectures from Baxter, Bayer, CSL Behring, and Pfizer. R.K. received research funding from Bayer and consulting or lecture fees from Bayer, BioMarin and Pfizer. S.G. received an unrestricted medical research grant from Sobi D.C. reported no competing interests M.M. has received honoraria for lecturing and grant reviewing from NovoNordisk, Takeda, Grifols and Sanofi.
Figures



References
-
- Nilsson I. M., Blomback M., and Ahlberg A., “Our Experience in Sweden With Prophylaxis on Haemophilia,” Bibliotheca Haematologica 34 (1970): 111–124. - PubMed

