Spermidine prevents iron overload-induced impaired bone mass by activating SIRT1/SOD2 signaling in senile rat model
- PMID: 40173181
- PMCID: PMC11966988
- DOI: 10.1080/13510002.2025.2485666
Spermidine prevents iron overload-induced impaired bone mass by activating SIRT1/SOD2 signaling in senile rat model
Abstract
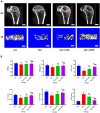
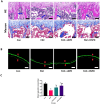
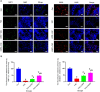
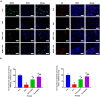
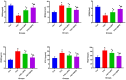
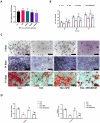
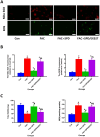
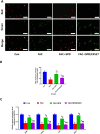
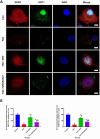
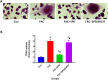
Spermidine (SPD) is an organic compound known for its powerful antioxidant stress and anti-aging properties, and whether SPD has the ability to reduce bone mass in elderly iron overload rats is unknown. The study aimed to assess SPD's impact on iron overload-induced bone loss in elderly rats. In our aged rat model, we found that iron overload negatively influences bone metabolism and remodeling, resulting in decreased bone mineral density and increased bone loss. However, SPD treatment effectively alleviated these harmful effects, as shown by reduced serum levels of MDA and increased SOD and GSH levels. Additionally, SPD-treated rats exhibited enhanced bone mass and higher expression of OC, BMP2, SIRT1, and SOD2 in their bones. Moreover, SPD restored the imbalance in bone metabolism by counteracting the inhibition of osteogenic differentiation and promoting osteoclast differentiation induced by iron overload in MC3T3-E1 and RAW264.7 cells affected by EX527. In summary, our findings suggest that SPD's antioxidant properties may exert anti-osteoporosis effects through activation of the SIRT1/SOD2 signaling pathway.
Keywords: Iron overload; MC3T3-E1; RAW264.7; SIRT1/SOD2 signaling pathway; bone mass; bone metabolism; oxidative stress; senile osteoporosis; spermidine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Protocatechualdehyde inhibits iron overload-induced bone loss by inhibiting inflammation and oxidative stress in senile rats.Int Immunopharmacol. 2024 Nov 15;141:113016. doi: 10.1016/j.intimp.2024.113016. Epub 2024 Aug 24. Int Immunopharmacol. 2024. PMID: 39182269
-
Niacin treatment prevents bone loss in iron overload osteoporotic rats via activation of SIRT1 signaling pathway.Chem Biol Interact. 2024 Jan 25;388:110827. doi: 10.1016/j.cbi.2023.110827. Epub 2023 Dec 9. Chem Biol Interact. 2024. PMID: 38081572
-
Silymarin prevents iron overload induced bone loss by inhibiting oxidative stress in an ovariectomized animal model.Chem Biol Interact. 2022 Oct 1;366:110168. doi: 10.1016/j.cbi.2022.110168. Epub 2022 Sep 7. Chem Biol Interact. 2022. PMID: 36087815
-
Paederosidic acid protect bone mass in lipopolysaccharide-treated rats by reducing oxidative stress and inflammatory.Int Immunopharmacol. 2024 Dec 25;143(Pt 2):113420. doi: 10.1016/j.intimp.2024.113420. Epub 2024 Oct 26. Int Immunopharmacol. 2024. PMID: 39490144
-
Melatonin prevents bone loss in osteoporotic rats with valproic acid treatment by anti-inflammatory and anti-oxidative stress.Int Immunopharmacol. 2024 Nov 15;141:112932. doi: 10.1016/j.intimp.2024.112932. Epub 2024 Aug 17. Int Immunopharmacol. 2024. PMID: 39154533
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
