Neural stimulation suppresses mTORC1-mediated protein synthesis in skeletal muscle
- PMID: 40173236
- PMCID: PMC11963989
- DOI: 10.1126/sciadv.adt4955
Neural stimulation suppresses mTORC1-mediated protein synthesis in skeletal muscle
Abstract
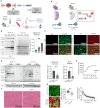
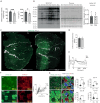
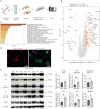
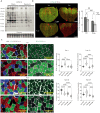
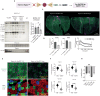
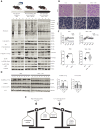
Skeletal muscle fibers are classified as glycolytic or oxidative, with differing susceptibilities to muscle wasting. However, the intracellular signaling pathways regulating fiber-specific muscle trophism remain unclear because of a lack of experimental models measuring protein synthesis. We developed a mouse model overexpressing a mutated transfer RNA synthetase in muscle fibers, enabling specific protein labeling using an artificial methionine substitute, which can be revealed through click chemistry. This model revealed that denervation increases protein labeling in oxidative muscle fibers through mammalian target of rapamycin complex 1 (mTORC1) activation, while deleting the mTORC1 scaffold protein Raptor reduces labeling in glycolytic fibers. On the other hand, increased muscle activity acutely decreases protein synthesis, accompanied by reduced mTORC1 signaling, glycogen depletion, and adenosine 5'-monophosphate kinase activation. Our findings identify nerve activity as an inhibitory signal for mTORC1-dependent protein synthesis in skeletal muscle, enhancing the understanding of fiber-specific responses to exercise and pathological conditions.
Figures
References
-
- Blaauw B., Schiaffino S., Reggiani C., Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 3, 1645–1687 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

