The severity of SLC1A2-associated neurodevelopmental disorders correlates with transporter dysfunction
- PMID: 40174554
- PMCID: PMC11999296
- DOI: 10.1016/j.ebiom.2025.105648
The severity of SLC1A2-associated neurodevelopmental disorders correlates with transporter dysfunction
Abstract
Background: Excitatory amino acid transporter 2 (EAAT2) is the predominant glutamate transporter and a key mediator of excitatory neurotransmission in the human brain. Here we present a cohort of 18 individuals harbouring 13 different SLC1A2 variants, who all present with neurodevelopmental impairment with variable symptoms and disease severities, and we delineate the impact of these variants on EAAT2 function.
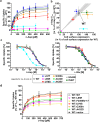
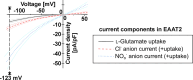
Methods: The consequences of nine novel missense SLC1A2 variants for expression, transport and anion channel properties of EAAT2 expressed in mammalian cells were characterized by confocal microscopy, enzyme-linked immunosorbent and [3H]-D-aspartate uptake assays, and electrophysiological recordings.
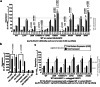
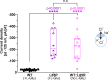
Findings: Ten of the 13 SLC1A2 variants mediated significant changes to EAAT2 expression and/or function. These molecular phenotypes were classified into three categories: overall loss-of-function (F249Sfs∗17, A432D, A439V, c.1421+1G>C), mild gain-of-anion-channel function (I276S, G360A), and mixed loss-of-transport/gain-of-anion-channel function (G82R, L85R, L85P, P289R). In contrast, L37P, H542R and I546T did not mediate significant changes to EAAT2 expression or function. Although specific clinical outcomes in individuals carrying variants within each category varied somewhat, the three categories overall translated into distinct clinical phenotypes in terms of phenotypic traits and severity.
Interpretation: The observed associations between functional effects and clinical phenotypes produced by these variants offer valuable insights for future predictions of progression and severity of SLC1A2-associated neurodevelopmental disorders. Furthermore, these associations between variant-induced changes in EAAT2 function and phenotypic traits could assist in tailoring personalized treatments of these disorders.
Funding: This work was funded by the German Ministry of Education and Research and by the Lundbeck Foundation.
Keywords: Epilepsy; Excitatory amino acid transporter 2 (EAAT2); Genetic variants; Patch clamp; Phenotyping; SLC1A2; Transporter uptake.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests RSM has received consulting fees from UCB, Orion, Saniona and Immedica, and speaker fees from EISAI, Angelini Pharma, Jazz Pharmaceuticals, Orion and UCB. The remaining authors declare no competing interests.
Figures








References
-
- Kwan C., Kang W., Kim E., Belliveau S., Frouni I., Huot P. Metabotropic glutamate receptors in Parkinson’s disease. Int Rev Neurobiol. 2023;168:1–31. - PubMed
-
- Danbolt N.C. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. - PubMed
-
- Vandenberg R.J., Ryan R.M. Mechanisms of glutamate transport. Physiol Rev. 2013;93(4):1621–1657. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

