Neutrophilic inflammation in bronchiectasis
- PMID: 40174958
- PMCID: PMC11962982
- DOI: 10.1183/16000617.0179-2024
Neutrophilic inflammation in bronchiectasis
Abstract
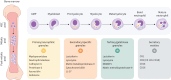
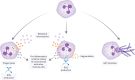
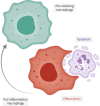
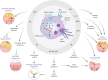
Noncystic fibrosis bronchiectasis, hereafter referred to as bronchiectasis, is a chronic, progressive lung disease that can affect people of all ages. Patients with clinically significant bronchiectasis have chronic cough and sputum production, as well as recurrent respiratory infections, fatigue and impaired health-related quality of life. The pathophysiology of bronchiectasis has been described as a vicious vortex of chronic inflammation, recurring airway infection, impaired mucociliary clearance and progressive lung damage that promotes the development and progression of the disease. This review describes the pivotal role of neutrophil-driven inflammation in the pathogenesis and progression of bronchiectasis. Delayed neutrophil apoptosis and increased necrosis enhance dysregulated inflammation in bronchiectasis and failure to resolve this contributes to chronic, sustained inflammation. The excessive release of neutrophil serine proteases, such as neutrophil elastase, cathepsin G and proteinase 3, promotes a protease-antiprotease imbalance that correlates with increased inflammation in bronchiectasis and contributes to disease progression. While there are currently no licensed therapies to treat bronchiectasis, this review will explore the evolving evidence for neutrophilic inflammation as a novel treatment target with meaningful clinical benefits.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: J.D. Chalmers reports receiving grants and personal fees from AstraZeneca, Boehringer Ingelheim, GSK, Insmed Incorporated and Zambon; a grant from Gilead; and personal fees from Chiesi and Novartis. M. Metersky reports receiving consulting fees from AN2 Therapeutics, Boehringer Ingelheim, Insmed Incorporated, Renovion, Tactile Inc. and Zambon. S. Aliberti reports receiving personal fees from AstraZeneca, Bayer Healthcare, Chiesi, GlaxoSmithKline, Grifols, Insmed Incorporated, Menarini, Zambon and ZetaCube; and grants from Chiesi, Fisher & Paykel and Insmed Incorporated, outside of the submitted work. L. Morgan reports receiving grants and personal fees from AstraZeneca, Boehringer Ingelheim, GSK, Insmed Incorporated, Novartis and Zambon. S. Fucile, M. Lauterio and P.P. McDonald are employees and shareholders in Insmed Incorporated.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
