SGK1 upregulation in GFAP+ neurons in the frontal association cortex protects against neuronal apoptosis after spinal cord injury
- PMID: 40175324
- PMCID: PMC11965300
- DOI: 10.1038/s41419-025-07542-y
SGK1 upregulation in GFAP+ neurons in the frontal association cortex protects against neuronal apoptosis after spinal cord injury
Abstract
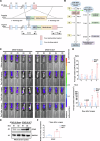
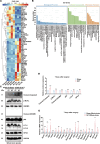
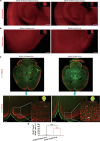
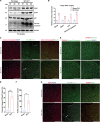
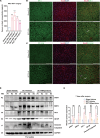
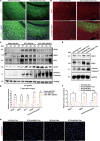
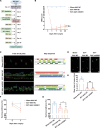
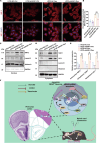
Spinal cord injury (SCI) casts devastating and long-lasting impacts on the well-being of patients. Cognitive deficits and emotional disorders are common in individuals with SCI, yet the underlying mechanisms are not completely understood. Astrogliosis and glial scar formation occur during the subacute phase post-injury, playing complicated roles in remyelination and neurite regrowth. Therefore, we constructed a GFAP-IRES-Venus-AkaLuc knock-in mouse model for the corresponding studies. Surprisingly, complete spinal cord transection (SCT) surgery led to earlier and more prominent augmentation of bioluminescence in the brain than in the spinal cord. Bulk RNA sequencing revealed the activation of apoptotic signaling and the upregulation of serum and glucocorticoid-regulated kinase 1 (SGK1). The pattern of GFAP signals changed throughout the brain after SCT, as indicated by tissue clearing and immunostaining. Specifically, GFAP signals were intensified in the frontal association cortex (FrA), an encephalic region involved in associative learning and recognition memory processes. Further exploration unraveled that intensified GFAP signals in the FrA were attributed to apoptotic neurons with SGK1 upregulation, which was induced by sustained high glucocorticoid levels after SCT. The introduction of SGK1 silencing vectors confirmed that SGK upregulation in these FrA neurons exerted anti-apoptotic effects through NRF2/HO-1 signaling. In addition, SGK1 knockdown in FrA neurons aggravated the post-SCI depressive-like behaviors. Thus, ectopic SGK1 expression designated for limbic neurons could serve as a promising therapeutic target for the future development of treatments for spinal cord injuries.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

