Cross-ancestry genome-wide association study identifies implications of SORL1 in cerebral beta-amyloid deposition
- PMID: 40175353
- PMCID: PMC11965558
- DOI: 10.1038/s41467-025-57751-4
Cross-ancestry genome-wide association study identifies implications of SORL1 in cerebral beta-amyloid deposition
Abstract
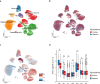
GWAS of Alzheimer's disease have been predominantly based on European ancestry cohorts with clinically diagnosed patients. Increasing the ancestral diversity of GWAS and focusing on imaging brain biomarkers for Alzheimer's disease may lead to the identification of new genetic loci. Here, we perform a GWAS on cerebral β-amyloid deposition measured by PET imaging in 3,885 East Asians and a cross-ancestry GWAS meta-analysis with data from 11,816 European participants. Our GWAS analysis replicates known loci (APOE4, CR1, and FERMT2) and identifies a novel locus near SORL1 that is significantly associated with β-amyloid deposition. Single-nucleus expression analysis shows that SORL1 is differentially expressed according to β-amyloid positivity in microglia. Our joint association analysis using the SORL1 lead variant (rs76490923) and the APOE4 allele demonstrates that the risk of β-amyloid deposition is reduced by up to 43.5% in APOE4 non-carriers and up to 55.6% in APOE4 carriers, according to the allelic dosage of the rs76490923 T allele. Our findings suggest that SORL1 may play an important role in the pathogenesis of Alzheimer's disease, particularly in relation to β-amyloid deposition.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Gatz, M. et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry63, 168–174 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RS-2023-00262527/National Research Foundation of Korea (NRF)
- RS-2019-NR040057/National Research Foundation of Korea (NRF)
- RS-2022-KH125557/Ministry of Health and Welfare (Ministry of Health, Welfare and Family Affairs)
- RS-2023-00247408/National Research Foundation of Korea (NRF)
- 2023-ER1001-01/Ministry of Health, Welfare and Family Affairs | Korea National Institute of Health (KNIH)
- 2023 Postdoctoral Research Program/Sungkyunkwan University (Sung Kyun Kwan University)
- RS-2020-KH106434/Ministry of Health and Welfare (Ministry of Health, Welfare and Family Affairs)
- RS-2024-00223277/National Research Foundation of Korea (NRF)
- RS-2021-II212068/Ministry of Science, ICT and Future Planning (MSIP)
- P30 AG066514/AG/NIA NIH HHS/United States
- 2024-ER1003-00/Ministry of Health, Welfare and Family Affairs | Korea National Institute of Health (KNIH)
LinkOut - more resources
Full Text Sources
Medical

