Systematic review and consensus conceptual model of meaningful symptoms and functional impacts in early Parkinson's Disease
- PMID: 40175369
- PMCID: PMC11965473
- DOI: 10.1038/s41531-025-00907-2
Systematic review and consensus conceptual model of meaningful symptoms and functional impacts in early Parkinson's Disease
Abstract
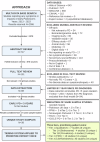
A comprehensive, patient-centered conceptual model of early Parkinson's is lacking and is greatly needed. A systematic review and meta-synthesis of qualitative and quantitative research was conducted by a multi-stakeholder taskforce using JBI Mixed Methods Review criteria and GRADE-CERQual standards for assessment of evidence. Over 340 symptoms and impacts were identified across ten symptom domains (Movement, Cognitive, Psychiatric, Sleep, Sensory, Speech, Digestive, Urinary, Sexual, Autonomic) and two impact domains (Physical and Psychosocial functioning). A wide range of motor and non-motor symptoms were present in early disease, with strongest support for tremor, dexterity, gait, stiffness, slow movements, cognitive, mood, and sleep alterations, urinary dysfunction, constipation, pain, and fatigue. These affected mobility, self-concept, coping, effort of living, interactions and important activities, with evidence of many understudied concepts. This model offers the most comprehensive catalogue of symptoms and impacts in Parkinson's to date and will support clinical practice and endpoint selection for therapeutic trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M. has received funding from Michael J. Fox Foundation for Parkinson’s Research (MJFF). G.T.S. is an employee of Rush University and has consulting and advisory board membership with honoraria for: Acadia Pharmaceuticals; Adamas Pharmaceuticals, Inc.; Biogen, Inc.; Ceregene, Inc.; CHDI Management, Inc.; the Cleveland Clinic Foundation; Ingenix Pharmaceutical Services (i3 Research); MedGenesis Therapeutix, Inc.; Neurocrine Biosciences, Inc.; Pfizer, Inc.; Tools-4-Patients; Ultragenyx, Inc.; and the Sunshine Care Foundation. He has received grants from and done research for: the National Institutes of Health, the Department of Defense, the Michael J. Fox Foundation for Parkinson’s Research, the Dystonia Coalition, CHDI, the Cleveland Clinic Foundation, the International Parkinson and Movement Disorder Society, and CBD Solutions, and has received honoraria from: the International Parkinson and Movement Disorder Society, the American Academy of Neurology, the Michael J. Fox Foundation for Parkinson’s Research, the FDA, the National Institutes of Health, and the Alzheimer’s Association. J.L.A. has received research support from the Michael J. Fox Foundation for Parkinson’s Research, Critical Path for Parkinson’s, NIH/NINDS, Biogen, the Huntington Study Group, and PhotoPharmics; received compensation as a consultant/steering committee/advisory board member from the Huntington Study Group, the Parkinson Study Group, AbbVie, VisualDx, BioSensics, Sana Biotechnology, Neuron23, Biohaven, and the Michael J. Fox Foundation for Parkinson’s Research; received honoraria for speaking from the Huntington Study Group, the Parkinson Study Group, American Neurological Association, Lundbeck, and the Ohio State University. J.R.M. has received research support from the NIH/NINR, Michael J. Fox Foundation for Parkinson’s Research (MJFF) and consulted for MJFF and Lundbeck HS. M.T. has received funding from Michael J. Fox Foundation for Parkinson’s Research (MJFF). T.M. is an employee of UCB Pharma. In the last 12 months T.S. has served as a consultant for AskBio, Amneal, Blue Rock Therapeutics, Critical Path for Parkinson’s Consortium (CPP), Denali, General Electric, Kyowa, Neuroderm/ MTPA, Prevail/ Lilly, Roche, Sanofi, Sinopia, Takeda and Vanqua Bio. T.S. served on the ad board for AskBio, Amneal, Biohaven, Denali, GAIN, General Electric, Kyowa, MJFF, Neuron23, Parkinson Study Group, Prevail/ Lilly, and Roche. T.S. has served as a member of the scientific advisory board of Koneksa, Neuroderm/ MTPA, Sanofi and UCB. T.S. has received research funding from Amneal, Biogen, Neuroderm, Prevail, Roche,UCB and is an investigator for NINDS, MJFF, Parkinson’s Foundation. D.W. has received funding from Michael J. Fox Foundation for Parkinson’s Research (MJFF). The remaining authors (Y.X., W.B., M.T., C.R., M.C., C.C., K.C., R.C., H.M., J.M., G.S., C.T., C.B., E.D., C.K., D.S.,) have no relevant conflict of interest to disclose.
Figures
References
-
- Sertkaya, A., Birkenbach, A., Berlind, A. & Eyraud, J. Examination of Clinical Trial Costs and Barriers for Drug Development. https://aspe.hhs.gov/reports/examination-clinical-trial-costs-barriers-d... (2014).
LinkOut - more resources
Full Text Sources