Structural mimicry of UM171 and neomorphic cancer mutants co-opts E3 ligase KBTBD4 for HDAC1/2 recruitment
- PMID: 40175372
- PMCID: PMC11965401
- DOI: 10.1038/s41467-025-58350-z
Structural mimicry of UM171 and neomorphic cancer mutants co-opts E3 ligase KBTBD4 for HDAC1/2 recruitment
Abstract
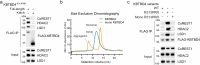
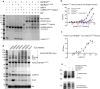
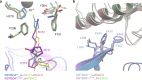
Neomorphic mutations and drugs can elicit unanticipated effects that require mechanistic understanding to inform clinical practice. Recurrent indel mutations in the Kelch domain of the KBTBD4 E3 ligase rewire epigenetic programs for stemness in medulloblastoma by recruiting LSD1-CoREST-HDAC1/2 complexes as neo-substrates for ubiquitination and degradation. UM171, an investigational drug for haematopoietic stem cell transplantation, was found to degrade LSD1-CoREST-HDAC1/2 complexes in a wild-type KBTBD4-dependent manner, suggesting a potential common mode of action. Here, we identify that these neomorphic interactions are mediated by the HDAC deacetylase domain. Cryo-EM studies of both wild-type and mutant KBTBD4 capture 2:1 and 2:2 KBTBD4-HDAC2 complexes, as well as a 2:1:1 KBTBD4-HDAC2-CoREST1 complex, at resolutions spanning 2.7 to 3.3 Å. The mutant and drug-induced complexes adopt similar structural assemblies requiring both Kelch domains in the KBTBD4 dimer for each HDAC2 interaction. UM171 is identified as a bona fide molecular glue binding across the ternary interface. Most strikingly, the indel mutation reshapes the same surface of KBTBD4 providing an example of a natural mimic of a molecular glue. Together, the structures provide mechanistic understanding of neomorphic KBTBD4, while structure-activity relationship (SAR) analysis of UM171 reveals analog S234984 as a more potent molecular glue for future studies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

