Photocatalytic deoxygenative Z-selective olefination of aliphatic alcohols
- PMID: 40175387
- PMCID: PMC11965276
- DOI: 10.1038/s41467-025-58469-z
Photocatalytic deoxygenative Z-selective olefination of aliphatic alcohols
Abstract
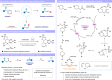
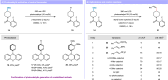
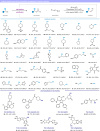
Alcohols are one of the most abundant functional groups in commercially available materials and biologically active compounds. Herein, we report a metal-free photocatalytic method for the deoxygenative Z-selective olefination of aliphatic alcohols. Key to this methodology is the radical olefination and isomerization of unstabilized open-shell species generated in situ by a catalytic reductive scission of benzoate esters. These processes are simultaneously orchestrated by a single phenothiazine photocatalyst via singlet and triplet excited states. Our protocol is distinguished by its wide substrate scope and broad applicability, even in the context of pharmaceuticals and saccharides. Given the mild and water-compatible conditions, our chemistry can also be utilized to functionalize DNA headpieces for DELs applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
Grants and funding
- TED2021-129833A-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- PID2020-113067GA-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- ED431G 2023/03/Xunta de Galicia
- ED481A-2023-178/Xunta de Galicia
LinkOut - more resources
Full Text Sources

