Sulforaphane protects developing neural networks from VPA-induced synaptic alterations
- PMID: 40175519
- PMCID: PMC12339368
- DOI: 10.1038/s41380-025-02967-5
Sulforaphane protects developing neural networks from VPA-induced synaptic alterations
Abstract
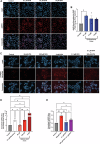
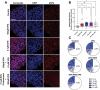
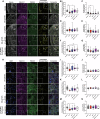
Prenatal brain development is particularly sensitive to chemicals that can disrupt synapse formation and cause neurodevelopmental disorders. In most cases, such chemicals increase cellular oxidative stress. For example, prenatal exposure to the anti-epileptic drug valproic acid (VPA), induces oxidative stress and synaptic alterations, promoting autism spectrum disorders (ASD) in humans and autism-like behaviors in rodents. Using VPA to model chemically induced ASD, we tested whether activation of cellular mechanisms that increase antioxidant gene expression would be sufficient to prevent VPA-induced synaptic alterations. As a master regulator of cellular defense pathways, the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) promotes expression of detoxification enzymes and antioxidant gene products. To increase NRF2 activity, we used the phytochemical and potent NRF2 activator, sulforaphane (SFN). In our models of human neurodevelopment, SFN activated NRF2, increasing expression of antioxidant genes and preventing oxidative stress. SFN also enhanced expression of genes associated with synapse formation. Consistent with these gene expression profiles, SFN protected developing neural networks from VPA-induced reductions in synapse formation. Furthermore, in mouse cortical neurons, SFN rescued VPA-induced reductions in neural activity. These results demonstrate the ability of SFN to protect developing neural networks during the vulnerable period of synapse formation, while also identifying molecular signatures of SFN-mediated neuroprotection that could be relevant for combatting other environmental toxicants.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Sealey LA, Hughes BW, Sriskanda AN, Guest JR, Gibson AD, Johnson-Williams L, et al. Environmental factors in the development of autism spectrum disorders. Environ Int. 2016;88:288–98. - PubMed
-
- Yenkoyan K, Mkhitaryan M, Bjørklund G. Environmental risk factors in autism spectrum disorder: a narrative review. Curr Med Chem. 2024;31:2345–60. - PubMed
MeSH terms
Substances
Grants and funding
- P30 ES025128/ES/NIEHS NIH HHS/United States
- R21 AT011371/AT/NCCIH NIH HHS/United States
- 1R21AT011371-01A1/U.S. Department of Health & Human Services | NIH | National Center for Complementary and Integrative Health (NCCIH)
- 2144912/National Science Foundation (NSF)
- P30ES025128/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

