The impact of ingestion of Bifidobacterium longum NCC3001 on perinatal anxiety and depressive symptoms: a randomized controlled trial
- PMID: 40175540
- PMCID: PMC11965328
- DOI: 10.1038/s41598-025-95651-1
The impact of ingestion of Bifidobacterium longum NCC3001 on perinatal anxiety and depressive symptoms: a randomized controlled trial
Abstract
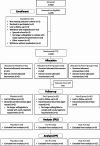
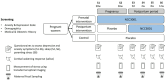
Perinatal mood disorders, including depression and anxiety, are common. Pregnant and lactating women often limit their use of medications, thus a safe and natural solution to improve mood would be welcomed. There is increasing evidence that probiotics such as Bifidobacterium longum NCC3001 can influence mental well-being of adults; however, their impact on mental health during pregnancy and after birth remains unknown. The current double-blind, placebo-controlled, randomized, 3-parallel-arm study (N = 184) evaluated the efficacy of orally consumed B. longum (BL) NCC3001 either during pregnancy and postpartum (from approximately 30 weeks' gestation until 12 weeks after delivery) or postpartum only (from birth until 12 weeks after delivery) compared to a placebo control group in reducing depressive and anxiety symptoms assessed by EPDS and STAI self-administered questionnaires in late pregnancy and across 12 weeks postpartum. Contrary to our hypothesis, we did not observe any differences between groups in mood outcomes. Mood scores showed large variability among participants, as well as notable fluctuations within individuals over the course of the study. Additionally, it should be noted that BL NCC3001 was not detected after the intervention in all of the intervention group participants. More research is needed to understand the underpinnings of perinatal mood disturbances and microbial changes, and whether probiotics could improve mood during this period.
Keywords: Gut-brain axis; Mood; Postpartum depression; Probiotics; Stress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Informed consent: Written informed consent was obtained from all subjects involved in the study. Institutional review board statement: The study was registered on 28/12/2020 at ClinicalTrials.gov (NCT04685252). The study was conducted in accordance with the Declaration of Helsinki and was approved by the A*STAR IRB (Ref No.: 2020-065). Competing interests: LRF, LL, MB, OS, FC, SK, NP, MV, GB and ISZ are employees of Société des Produits Nestlé S.A., Switzerland. SC is part of an academic consortium that has received grants from Société des Produits Nestlé S.A. for work unrelated to the present manuscript and has received reimbursement from the Expert Group on Inositol in Basic and Clinical Research (EGOI; a not-for-profit academic organization) and Nestlé Nutrition Institute for speaking at conferences. All other authors have no competing interest.
Figures
References
-
- Shorey, S. et al. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res.104, 235–248. 10.1016/j.jpsychires.2018.08.001 (2018). - PubMed
-
- Dennis, C. L., Falah-Hassani, K. & Shiri, R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry. 210, 315–323. 10.1192/bjp.bp.116.187179 (2017). - PubMed
-
- Rezaie-Keikhaie, K. et al. Systematic review and meta-analysis of the prevalence of the maternity blues in the postpartum period. J. Obstetric Gynecologic Neonatal Nursing: JOGNN. 49, 127–136. 10.1016/j.jogn.2020.01.001 (2020). - PubMed
-
- Chee, C. Y. et al. Confinement and other psychosocial factors in perinatal depression: a transcultural study in Singapore. J. Affect. Disord.89, 157–166. 10.1016/j.jad.2005.09.004 (2005). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

