The RAD52 double-ring remodels replication forks restricting fork reversal
- PMID: 40175552
- PMCID: PMC12360664
- DOI: 10.1038/s41586-025-08753-1
The RAD52 double-ring remodels replication forks restricting fork reversal
Abstract
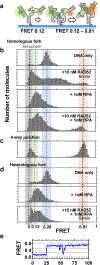
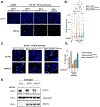
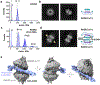
Human RAD52 is a multifunctional DNA repair protein involved in several cellular events that support genome stability, including protection of stalled DNA replication forks from excessive degradation1-4. In its gatekeeper role, RAD52 binds to and stabilizes stalled replication forks during replication stress, protecting them from reversal by SMARCAL1 motor3. The structural and molecular mechanism of the RAD52-mediated fork protection remains elusive. Here, using P1 nuclease sensitivity, biochemical and single-molecule analyses, we show that RAD52 dynamically remodels replication forks through its strand exchange activity. The presence of the single-stranded DNA binding protein RPA at the fork modulates the kinetics of the strand exchange without impeding the reaction outcome. Mass photometry and single-particle cryo-electron microscopy show that the replication fork promotes a unique nucleoprotein structure containing head-to-head arrangement of two undecameric RAD52 rings with an extended positively charged surface that accommodates all three arms of the replication fork. We propose that the formation and continuity of this surface is important for the strand exchange reaction and for competition with SMARCAL1.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








Update of
-
A double-ring of human RAD52 remodels replication forks restricting fork reversal.bioRxiv [Preprint]. 2024 Sep 18:2023.11.14.566657. doi: 10.1101/2023.11.14.566657. bioRxiv. 2024. Update in: Nature. 2025 May;641(8062):512-519. doi: 10.1038/s41586-025-08753-1. PMID: 38014173 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

