Bifidobacteria support optimal infant vaccine responses
- PMID: 40175554
- PMCID: PMC12058517
- DOI: 10.1038/s41586-025-08796-4
Bifidobacteria support optimal infant vaccine responses
Abstract
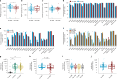
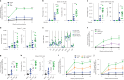
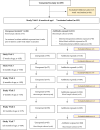
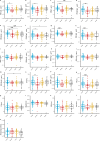
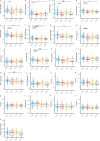
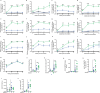
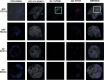
Accumulating evidence indicates that antibiotic exposure may lead to impaired vaccine responses1-4; however, the mechanisms underlying this association remain poorly understood. Here we prospectively followed 191 healthy, vaginally born, term infants from birth to 15 months, using a systems vaccinology approach to assess the effects of antibiotic exposure on immune responses to vaccination. Exposure to direct neonatal but not intrapartum antibiotics was associated with significantly lower antibody titres against various polysaccharides in the 13-valent pneumococcal conjugate vaccine and the Haemophilus influenzae type b polyribosylribitol phosphate and diphtheria toxoid antigens in the combined 6-in-1 Infanrix Hexa vaccine at 7 months of age. Blood from infants exposed to neonatal antibiotics had an inflammatory transcriptional profile before vaccination; in addition, faecal metagenomics showed reduced abundance of Bifidobacterium species in these infants at the time of vaccination, which was correlated with reduced vaccine antibody titres 6 months later. In preclinical models, responses to the 13-valent pneumococcal conjugate vaccine were strongly dependent on an intact microbiota but could be restored in germ-free mice by administering a consortium of Bifidobacterium species or a probiotic already widely used in neonatal units. Our data suggest that microbiota-targeted interventions could mitigate the detrimental effects of early-life antibiotics on vaccine immunogenicity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.J.L., S.J.B., F.J.R. and M.A.L. have received funding from GSK related to this area of research. D.J.L. and H.S.M. are consultants for GPN Vaccines (Australia). H.S.M. is an investigator on sponsored vaccine trials funded by Iliad and Pfizer. H.S.M. has received institutional funding for investigator-led studies from Pfizer, Sanofi-Aventis and Seqiris. P.R. has received institutional funding for investigator-led grants from Merck and Sanofi outside this work. His institution receives funding for his participation in vaccine scientific advisory boards from Pfizer, Merck, GSK, GPN Vaccines (Australia) and Sanofi-Aventis unrelated to this work. He is an investigator on sponsored vaccine trials funded by Iliad, GSK, Merck, Sanofi, Dynavax and Pfizer. He is coinventor on a patent for a new bacteriotherapy for treating or preventing respiratory infection unrelated to this work. The other authors declare no competing interests.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

