Experience of people with Biochemical Genetic Disorders and their families accessing Genetic Counselling and Genetic Testing in the Irish Republic
- PMID: 40175825
- PMCID: PMC12401831
- DOI: 10.1007/s12687-025-00791-6
Experience of people with Biochemical Genetic Disorders and their families accessing Genetic Counselling and Genetic Testing in the Irish Republic
Abstract
Background: National and international reports recommend that genetic counselling should be made available to parents of children living with inherited rare diseases; and to patients themselves upon turning 16-18 years old. Long wait times of up to two years for genetic counselling through Children's Health Ireland contributed to a lack of accessibility for adult patients with inherited metabolic disorders (IMDs). At the time of the study, the National Centre for Inherited Metabolic Disorders (NCIMD) Mater, which takes care of ~ 1400 adult patients with genetic disorders primarily affecting biochemical pathways, did not have direct access to a genetic counsellor.
Objectives and methods: An online survey was conducted to investigate the genetic testing and counselling experiences of adult patients with rare IMDs and their families within the Republic of Ireland.
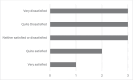
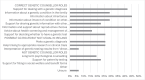
Results: The NCIMD-Mater survey highlighted a lack of patient knowledge of and access to genetic counselling services; with some patients unaware of and others incorrectly understanding the role of genetic counselling. Most patients who underwent genetic testing were tested by a non-genetic healthcare professional. Satisfaction levels of genetic counselling services were mixed with some patients reporting delaying personal life and family plans due to wait times for genetic counselling.
Conclusion: This study highlights deficiencies in the genetic testing and counselling experience of Irish IMD patients. Embedding genetic counselling into multidisciplinary IMD teams would increase access to genetics education for patients and families and improve the clinical service. This study may be utilized to measure the impact of integrating genetic counsellors into NCIMD-Mater.
Keywords: Background; Genetic counselling; Genetic testing; Patient experience; Rare metabolic disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations: Ceri Arnott, Alana Ward, Deborah Lambert, Dearbhla Butterly, Vicky McGrath, Sally Ann Lynch and James O’Byrne declare that they have no conflict of interest. The funding agency had no role or influence in design, analysis or reporting of this research. Financial support for Rare Diseases Ireland’s advocacy work is provided by several industry partners who have an interest in rare conditions but who have no role or influence in design, analysis or reporting of this research. Compliance with Ethics Guidelines: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. Approval for this survey was obtained from the MMUH Research Ethics committee (IRB Reference No: 1/378/2275). This article does not contain any studies with animal subjects performed by the any of the authors. Competing Interests: The authors declare no competing interests.
Figures

References
-
- Abrams DJ, Geier MR (2006) A comparison of patient satisfaction with telehealth and on-site consultations: A pilot study for prenatal genetic counseling. J Genet Couns 15(3):199–205. 10.1007/s10897-006-9020-0 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

