Combining treatment effects from mixed populations in meta-analysis: a review of methods
- PMID: 40175893
- PMCID: PMC11963434
- DOI: 10.1186/s12874-025-02507-3
Combining treatment effects from mixed populations in meta-analysis: a review of methods
Abstract
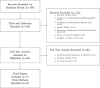
Background: Meta-analysis is a useful method for combining evidence from multiple studies to detect treatment effects that could perhaps not be identified in a single study. While traditionally meta-analysis has assumed that populations of included studies are comparable, over recent years the development of precision medicine has led to identification of predictive genetic biomarkers which has resulted in trials conducted in mixed biomarker populations. For example, early trials may be conducted in patients with any biomarker status with no subgroup analysis, later trials may be conducted in patients with any biomarker status and subgroup analysis, and most recent trials may be conducted in biomarker-positive patients only. This poses a problem for traditional meta-analysis methods which rely on the assumption of somewhat comparable populations across studies. In this review, we provide a background to meta-analysis methods allowing for synthesis of data with mixed biomarker populations across trials.
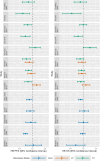
Methods: For the methodological review, PubMed was searched to identify methodological papers on evidence synthesis for mixed populations. Several identified methods were applied to an illustrative example in metastatic colorectal cancer.
Results: We identified eight methods for evidence synthesis of mixed populations where three methods are applicable to pairwise meta-analysis using aggregate data (AD), three methods are applicable to network meta-analysis using AD, and two methods are applicable to network meta-analysis using AD and individual participant data (IPD). The identified methods are described, including a discussion of the benefits and limitations of each method.
Conclusions: Methods for synthesis of data from mixed populations are split into methods which use (a) AD, (b) IPD, and (c) both AD and IPD. While methods which utilise IPD achieve superior statistical qualities, this is at the expense of ease of access to the data. Furthermore, it is important to consider the context of the decision problem in order to select the most appropriate modelling framework.
Keywords: Biomarker; Meta-analysis; Subgroup.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: SB is a member of the NICE Decision Support Unit (DSU) and the NICE Guidelines Technical Support Unit (TSU). She has served as a paid consultant to NICE, CROs and pharmaceutical industry providing unrelated methodological advice, has received payments for educational events from Roche and the University of Bristol, funding to attend conferences from CROs and Roche, research funding from European Federation of Pharmaceutical Industries & Associations (EEPIA) and Johnson & Johnson and research support in kind from AstraZeneca and Roche. SG is an employee of F. Hoffman-La Roche AG and holds shares of F. Hoffman-La Roche AG, Novartis AG, and Sandoz Group AG. LW and SH have no competing interests to declare.
Figures

References
-
- Tajik P, Zwinderman AH, Mol BW, Bossuyt PM. Trial Designs for Personalizing Cancer Care: A Systematic Review and ClassificationTrial Designs for Personalizing Cancer Care. Clin Cancer Res. 2013;19(17):4578–88. - PubMed
-
- Twomey JD, Brahme NN, Zhang B. Drug-biomarker co-development in oncology-20 years and counting. Drug Resist Updat. 2017;30:48–62. - PubMed
-
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34. - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, De Braud F, et al. Fluorouracil leucovorin and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71. - PubMed
-
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

