Rutin ameliorates stress-induced blood‒brain barrier dysfunction and cognitive decline via the endothelial HDAC1‒Claudin-5 axis
- PMID: 40176114
- PMCID: PMC11967129
- DOI: 10.1186/s12987-025-00639-8
Rutin ameliorates stress-induced blood‒brain barrier dysfunction and cognitive decline via the endothelial HDAC1‒Claudin-5 axis
Abstract
Background: Emerging evidence suggests that chronic stress compromises blood‒brain barrier (BBB) integrity by disrupting brain microvascular endothelial cells (BMECs), contributing to the development of cognitive impairments. Thus, targeting the BBB is expected to be a promising treatment strategy. The biological function of rutin has been investigated in neurological disorders; however, its regulatory role in stress-induced BBB damage and cognitive decline and the underlying mechanisms remain elusive.
Methods: In a chronic unpredictable mild stress (CUMS) mouse model, a fluorescent dye assay and behavioral tests, including a novel object recognition test and Morris water maze, were performed to evaluate the protective effects of rutin on BBB integrity and cognition. The effects of rutin on BMEC function were also investigated in hCMEC/D3 cells (a human brain microvascular endothelial cell line) in vitro. Furthermore, the molecular mechanisms by which rutin restores BBB endothelium dysfunction were explored via RNA-seq, quantitative real-time PCR, western blotting, immunofluorescence and chromatin immunoprecipitation. Finally, biotinylated tumor necrosis factor-α (TNF-α) was employed to test the influence of rutin on the ability of circulating TNF-α to cross the BBB.
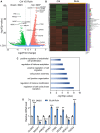
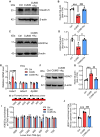
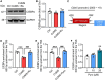
Results: We identified that rutin attenuated BBB hyperpermeability and cognitive impairment caused by the 8-week CUMS procedure. Moreover, rutin promoted the proliferation, migration and angiogenesis ability of BMECs, and the integrity of the cellular monolayer through positively regulating the expression of genes involved. Furthermore, rutin impeded histone deacetylase 1 (HDAC1) recruitment and stabilized H3K27ac to increase Claudin-5 protein levels. Ultimately, normalization of the hippocampal HDAC1‒Claudin-5 axis by rutin blocked the infiltration of circulating TNF-α into the brain parenchyma and alleviated neuroinflammation.
Conclusions: This work establishes a protective role of rutin in regulating BMEC function and BBB integrity, and reveals that rutin is a potential drug candidate for curing chronic stress-induced cognitive deficits.
Keywords: BBB; BMEC function; Claudin–5; H3K27ac modification; Rutin; Stress–induced cognitive decline.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments and experimental procedures were authorized by the Animal Care and Use Committee at the AMS (IACUC-DWZX-2022–738). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Saeedi M, Rashidy-Pour A. Association between chronic stress and Alzheimer’s disease: therapeutic effects of Saffron. Biomed Pharmacother. 2021;133:110995. - PubMed
-
- Lupien SJ, Juster RP, Raymond C, Marin MF. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol. 2018;49:91–105. - PubMed
-
- Escher CM, Sannemann L, Jessen F. Stress and Alzheimer’s disease. J Neural Transm. 2019;126:1155–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

