Peptide-Modified Lipid Nanoparticles Boost the Antitumor Efficacy of RNA Therapeutics
- PMID: 40176316
- PMCID: PMC12004924
- DOI: 10.1021/acsnano.4c14625
Peptide-Modified Lipid Nanoparticles Boost the Antitumor Efficacy of RNA Therapeutics
Abstract
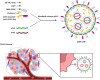
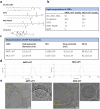
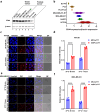
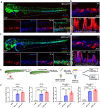
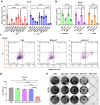
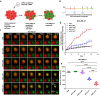
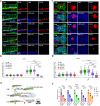
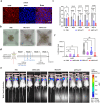
RNA therapeutics offer a promising approach to cancer treatment by precisely regulating cancer-related genes. While lipid nanoparticles (LNPs) are currently the most advanced nonviral clinically approved vectors for RNA therapeutics, their antitumor efficacy is limited by their unspecific hepatic accumulation after systemic administration. Thus, there is an urgent need to enhance the delivery efficiency of LNPs to target tumor-residing tissues. Here, we conjugated the cluster of differentiation 44 (CD44)-specific targeting peptide A6 (KPSSPPEE) to the cholesterol of LNPs via PEG, named AKPC-LNP, enabling specific tumor delivery. This modification significantly improved delivery to breast cancer cells both in vitro and in vivo, as shown by flow cytometry and confocal microscopy. We further used AKPC-siYT to codeliver siRNAs targeting the transcriptional coactivators YAP and TAZ, achieving potent gene silencing and increased cell death in both 2D cultures and 3D tumor spheroids, outperforming unmodified LNPs. In a breast tumor cell xenografted zebrafish model, systemically administered AKPC-siYT induced robust silencing of YAP/TAZ and downstream genes and significantly enhanced tumor suppression compared to unmodified LNPs. Additionally, AKPC-siYT effectively reduced proliferation in prostate cancer organoids and tumor growth in a patient-derived xenograft (PDX) model. Overall, we developed highly efficient AKPC-LNPs carrying RNA therapeutics for targeted cancer therapy.
Keywords: CD44; YAP/TAZ siRNA; lipid nanoparticles; patient-derived PDX; tumor targeting; zebrafish.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Efficient siRNA Delivery by Lipid Nanoparticles Modified with a Nonstandard Macrocyclic Peptide for EpCAM-Targeting.Mol Pharm. 2017 Oct 2;14(10):3290-3298. doi: 10.1021/acs.molpharmaceut.7b00362. Epub 2017 Aug 25. Mol Pharm. 2017. PMID: 28789523
-
Dual targeted lipid nanoparticles for enhanced DNA delivery and transfection of breast cancer cells.Eur J Pharm Biopharm. 2025 Apr;209:114674. doi: 10.1016/j.ejpb.2025.114674. Epub 2025 Feb 21. Eur J Pharm Biopharm. 2025. PMID: 39988265
-
Enhanced Delivery of siRNA to Retinal Ganglion Cells by Intravitreal Lipid Nanoparticles of Positive Charge.Mol Pharm. 2021 Jan 4;18(1):377-385. doi: 10.1021/acs.molpharmaceut.0c00992. Epub 2020 Dec 9. Mol Pharm. 2021. PMID: 33295773
-
Lipid‑based nanoparticles for the therapeutic delivery of non‑coding RNAs in breast cancer (Review).Oncol Rep. 2020 Dec;44(6):2353-2363. doi: 10.3892/or.2020.7791. Epub 2020 Oct 5. Oncol Rep. 2020. PMID: 33125103 Review.
-
Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications.Adv Drug Deliv Rev. 2023 Sep;200:114990. doi: 10.1016/j.addr.2023.114990. Epub 2023 Jul 7. Adv Drug Deliv Rev. 2023. PMID: 37423563 Review.
References
-
- Rosenblum D.; Gutkin A.; Kedmi R.; Ramishetti S.; Veiga N.; Jacobi A. M.; Schubert M. S.; Friedmann-Morvinski D.; Cohen Z. R.; Behlke M. A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6 (47), eabc945010.1126/sciadv.abc9450. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

