Altered Brain-Behavior Association During Resting State is a Potential Psychosis Risk Marker
- PMID: 40176373
- PMCID: PMC12245128
- DOI: 10.1002/advs.202405700
Altered Brain-Behavior Association During Resting State is a Potential Psychosis Risk Marker
Abstract
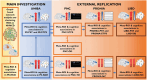
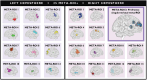
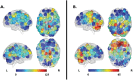
Alterations in cognitive and neuroimaging measures in psychosis may reflect altered brain-behavior interactions patterns accompanying the symptomatic manifestation of the disease. Using graph connectivity-based approaches, we tested the brain-behavior association between cognitive functioning and functional connectivity at different stages of psychosis. We collected resting-state fMRI of 204 neurotypical controls (NC) in two independent cohorts, 43 patients with chronic psychosis (PSY), and 22 subjects with subthreshold psychotic symptoms (STPS). In NC, we calculated graph connectivity metrics and tested their associations with neuropsychological scores. Replicable associations were tested in PSY and STPS and externally validated in three cohorts of 331, 371, and 232 individuals, respectively. NC showed a positive correlation between the degree centrality of a right prefrontal-cingulum-striatal circuit and total errors on Wisconsin Card Sorting Test. Conversely, PSY and STPS showed negative correlations. External replications confirmed both associations while highlighting the heterogeneity of STPS. Group differences in either centrality or cognition alone were not equally replicable. In four independent cohorts totaling 1,203 participants, we identified a replicable alteration of the brain-behavior association in different stages of psychosis. These results highlight the high replicability of multimodal markers and suggest the opportunity for longitudinal investigations that may test this marker for early risk identification.
Keywords: at risk mental states; brain networks; brain‐behavior relationship; chronic psychosis; executive function; neuropsychology.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
A.B. received consulting fees from Biogen and lecture fees from Otsuka, Janssen, and Lundbeck. G.B. reported receiving personal fees from Lundbeck outside the submitted work. N.K. received honoraria for talks presented at education meetings organized by Otsuka/Lundbeck. D.R.W. serves on the Scientific Advisory Boards of Sage Therapeutics and Pasithea Therapeutics. G.P. received lecture fees from Lundbeck. All other authors report no biomedical financial interests or potential conflicts of interest. The funding organizations stated above were not involved in the design and realization of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures


References
-
- a) da Silva Castanheira J., Orozco Perez H. D., Misic B., Baillet S., Nat. Commun. 2021, 12, 5713; - PMC - PubMed
- b) Finn E. S., Shen X., Scheinost D., Rosenberg M. D., Huang J., Chun M. M., Papademetris X., Constable R. T., Nat. Neurosci. 2015, 18, 1664; - PMC - PubMed
- c) Miranda‐Dominguez O., Mills B. D., Carpenter S. D., Grant K. A., Kroenke C. D., Nigg J. T., Fair D. A., PLoS One 2014, 9, 111048; - PMC - PubMed
- d) Valizadeh S. A., Liem F., Mérillat S., Hänggi J., Jäncke L., Sci. Rep. 2018, 8, 5611; - PMC - PubMed
- e) Vogt N., Nat. Methods 2015, 12, 1112. - PubMed
-
- a) Genon S., Eickhoff S. B., Kharabian S., Nat. Rev. Neurosci. 2022, 23, 307; - PubMed
- b) Kanai R., Rees G., Nat. Rev. Neurosci. 2011, 12, 231; - PubMed
- c) Mohr P. N., Nagel I. E., J. Neurosci. 2010, 30, 7755; - PMC - PubMed
- d) Shen X., Finn E. S., Scheinost D., Rosenberg M. D., Chun M. M., Papademetris X., Constable R. T., 2017, 12, 506; - PMC - PubMed
- e) Yarkoni T., Braver T. S., Gray J. R., Green L., J. Exp. Anal. Behav. 2005, 84, 537. - PMC - PubMed
-
- Lissek S., Tegenthoff M., Behav. Brain Res. 2021, 412, 113413. - PubMed
MeSH terms
Grants and funding
- P2022HNBJX/European Union - NextGenerationEU funding within the PRIN 2022 PNRR initiative
- 2021 Helmholtz Information and Data science Academy Grant
- European Union funding within the MUR PNRR Extended Partnership initiative on Neuroscience and Neuropharmacology (Project no. PE00000006 CUP H93C22000660006 "MNESYS, A multiscale integrated approach to the study of the nervous system in health and disease"
- T32 MH122394/MH/NIMH NIH HHS/United States
- Collaboration Grant from ITEL TELECOMUNICAZIONI srl
- 95-M-0150/Intramural Research Program of the NIMH
- No 964874 - REALMENT/European Union's Horizon 2020 research and innovation program under the grant agreement
- Complementary National Plan PNC-I.1 "Research initiatives for innovative technologies and pathways in the health and welfare sector" D.D. 931 of 06/06/2022, DARE - DigitAl lifelong pRevEntion initiative, code PNC0000002, CUP B53C22006420001
- 2017K2NEF4/Research Projects of National Relevance (PRIN) 2017 grant
- Apulian regional government for the project: "Early Identification of Psychosis Risk"
LinkOut - more resources
Full Text Sources
Medical
