Improved Clinical Outcomes With Elexacaftor/Tezacaftor/Ivacaftor in Patients With Cystic Fibrosis and Advanced Lung Disease: Real-World Evidence From an Italian Single-Center Study
- PMID: 40176392
- PMCID: PMC11965699
- DOI: 10.1002/prp2.70083
Improved Clinical Outcomes With Elexacaftor/Tezacaftor/Ivacaftor in Patients With Cystic Fibrosis and Advanced Lung Disease: Real-World Evidence From an Italian Single-Center Study
Abstract
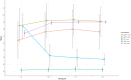
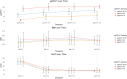
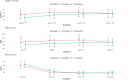
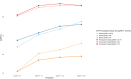
The combination of Elexacaftor/Tezacaftor/Ivacaftor (ETI) has resulted in a significant improvement in lung function and global clinical parameters, which have not been previously achieved with other CFTR modulators. However, there is a paucity of evidence in the literature on the long-term use of ETI in adolescents and patients with severe pulmonary impairment. Furthermore, the response to ETI may differ between homozygotes and heterozygotes, as well as between naïve patients and those previously treated with other CFTR modulators. A retrospective study was conducted to examine changes in percent predicted forced expiratory volume in 1 s (ppFEV1), body-mass index (BMI), and sweat chloride concentration (SwCl) at baseline and at 6, 12 and 24 months after the initiation of ETI. Secondary outcomes included the number of pulmonary exacerbations, Cystic Fibrosis Questionnaire-Revised (CFQ-R) score, adverse events, mortality and transplantation rates. 139 subjects were included and followed up for up to 2 years after starting ETI. The results demonstrated a significant improvement in ppFEV1 and BMI after 12 months of therapy (respectively, 16%, p < 0.001; +1.5 kg/m2, p = 0.005), with a slight decline in the values after 24 months. This effect was independent of genotype and showed a different degree of response in naïve subjects compared to patients previously treated with other CFTR modulators. SwCl decreased from 84 to 37 mmol/L over 24 months (p < 0.001). 58.3% reduction of PEx rate was observed compared to the number of exacerbations prior to ETI. Overall, lung function, SwCl, PEx rate, CFQ-R scores and BMI improved after 24 months of ETI treatment. ETI was well tolerated, and none of the patients interrupted the treatment due to toxicity.
Keywords: CFTR; Elexacaftor‐Tezacaftor‐Ivacaftor; cystic fibrosis; effectiveness; safety.
© 2025 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Heijerman H. G. M., McKone E. F., Downey D. G., et al., “Efficacy and Safety of the Elexacaftor Plus Tezacaftor Plus Ivacaftor Combination Regimen in People With Cystic Fibrosis Homozygous for the F508del Mutation: A Double‐Blind, Randomised, Phase 3 Trial,” Lancet 394 (2019): 1940–1948, 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

