NUPR1 Promotes Radioresistance in Colorectal Cancer Cells by Inhibiting Ferroptosis
- PMID: 40176685
- PMCID: PMC11965884
- DOI: 10.1111/jcmm.70519
NUPR1 Promotes Radioresistance in Colorectal Cancer Cells by Inhibiting Ferroptosis
Abstract
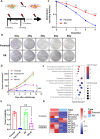
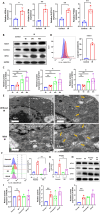
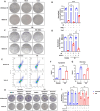
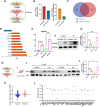
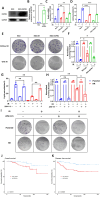
Radioresistance is a major clinical challenge and the underlying mechanism has not been thoroughly elucidated. In this study, a radioresistant (RR) cell line is established to explore the transcriptomic signatures of radioresistance in colorectal cancer (CRC). KEGG enriched pathway analysis demonstrated that ferroptosis is inactivated in RR cells. Further detection confirmed that radiotherapy can promote ferroptosis, and ferroptosis inactivation is one of the hallmarks of radioresistance in CRC. What's more, induction of ferroptosis can restore the radiosensitivity of CRC cells. Then, we performed RNA sequencing to compare gene expression between parental and RR cells, and cells pretreated with or without RSL3. Via high-throughput screening, NUPR1 was identified as a potential candidate for ferroptosis-mediated radioresistance in CRC. CRC cells can acquire radiation resistance by NUPR1-mediated ferroptosis suppression in the NUPR1-overexpressing cell line. More importantly, ZZW-115, an NUPR1 inhibitor, can sensitise RR cells to radiotherapy. Overall, our findings identify ferroptosis inactivation linked with resistance to radiotherapy. Besides, NUPR1 can promote radiation resistance by inhibiting ferroptosis, and targeting NUPR1 may be a potential strategy to relieve radioresistance associated with ferroptosis in CRC.
Keywords: NUPR1; colorectal cancer; ferroptosis; radiotherapy resistance.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
LncRNA FTX promotes colorectal cancer radioresistance through disturbing redox balance and inhibiting ferroptosis via miR-625-5p/SCL7A11 axis.World J Gastroenterol. 2025 Apr 28;31(16):104305. doi: 10.3748/wjg.v31.i16.104305. World J Gastroenterol. 2025. PMID: 40308806 Free PMC article.
-
CircPIAS1 promotes hepatocellular carcinoma progression by inhibiting ferroptosis via the miR-455-3p/NUPR1/FTH1 axis.Mol Cancer. 2024 May 28;23(1):113. doi: 10.1186/s12943-024-02030-x. Mol Cancer. 2024. PMID: 38802795 Free PMC article.
-
Transcriptional Regulation of NUPR1 by MYH11 Activates PI3 K/AKT and Promotes Bladder Cancer Progression Through Ferroptosis and M2 Polarization of Macrophages.Technol Cancer Res Treat. 2025 Jan-Dec;24:15330338241305434. doi: 10.1177/15330338241305434. Technol Cancer Res Treat. 2025. PMID: 39962891 Free PMC article.
-
Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma.Cells. 2019 Nov 17;8(11):1453. doi: 10.3390/cells8111453. Cells. 2019. PMID: 31744261 Free PMC article. Review.
-
NUPR1 and its potential role in cancer and pathological conditions (Review).Int J Oncol. 2021 May;58(5):21. doi: 10.3892/ijo.2021.5201. Epub 2021 Mar 24. Int J Oncol. 2021. PMID: 33760183 Review.
References
-
- “Erratum: Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 70, no. 4 (2020): 313. - PubMed
-
- Sauer R., Liersch T., Merkel S., et al., “Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO‐94 Randomized Phase III Trial After a Median Follow‐Up of 11 Years,” Journal of Clinical Oncology 30, no. 16 (2012): 1926–1933. - PubMed
-
- Bahadoer R. R., Dijkstra E. A., van Etten B., et al., “Short‐Course Radiotherapy Followed by Chemotherapy Before Total Mesorectal Excision (TME) Versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open‐Label, Phase 3 Trial,” Lancet Oncology 22, no. 1 (2021): 29–42, 10.1016/S1470-2045(20)30555-6. - DOI - PubMed
-
- Ngan S. Y., Burmeister B., Fisher R. J., et al., “Randomized Trial of Short‐Course Radiotherapy Versus Long‐Course Chemoradiation Comparing Rates of Local Recurrence in Patients With T3 Rectal Cancer: Trans‐Tasman Radiation Oncology Group Trial 01.04,” Journal of Clinical Oncology 30, no. 31 (2012): 3827–3833. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

