Effectiveness and safety of tenofovir alafenamide/emtricitabine/bictegravir as a first-line regimen in people with HIV: A retrospective observational study
- PMID: 40176857
- PMCID: PMC11964757
- DOI: 10.1016/j.ijregi.2025.100622
Effectiveness and safety of tenofovir alafenamide/emtricitabine/bictegravir as a first-line regimen in people with HIV: A retrospective observational study
Abstract
Objectives: To assess the effectiveness and safety of tenofovir alafenamide/emtricitabine/bictegravir (TAF/FTC/BIC) in patients newly diagnosed with HIV (PWH) in a non-experimental setting.
Methods: We conducted a single-center, retrospective observational study that included all newly diagnosed PWH treated with TAF/FTC/BIC at our institution. Virological failure was defined as two consecutive HIV-RNA values of >50 cp/ml after 48 weeks of treatment. Reasons for TAF/FTC/BIC interruption were also collected. The durability of TAF/FTC/BIC was estimated using Kaplan-Meier curves.
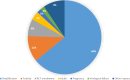
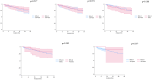
Results: A total of 236 PWH started TAF/FTC/BIC, with a median follow-up time of 13 months (interquartile range [IQR] 4-27 months). Most PWH were cisgender men (178/236, 75.4%) with a median age at diagnosis of 37 years (IQR 29-48) and a median cluster of differentiation 4 cell counts of 302 cells/mm³ (IQR 117-467). One protocol-defined virological failure was observed, without the development of drug resistance, resulting in an incidence of 3.1 per 1000 person-years of follow-up (95% confidence interval [CI] 0.8-17.3). Six (2.5%) PWH discontinued TAF/FTC/BIC because of toxicity. The estimated durabilities of TAF/FTC/BIC at 12 and 24 months were 84.8% (95% CI 78.6-89.3%) and 75.5% (95% CI 67.6-82.6%), respectively.
Conclusions: In our cohort of newly diagnosed PWH treated with TAF/FTC/BIC, the low occurrence of virological failure and discontinuation related to drug toxicities underscores the effectiveness and tolerability of the regimen.
Keywords: Advance naïve; Bictegravir; Durability; HIV; Single-tablet regimens.
© 2025 The Author(s).
Conflict of interest statement
Andrea Giacomelli reports consulting fees from Mylan and Jansen; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Gilead and ViiV; payment for expert testimony from Jansen; support for attending meetings and/or travel from Gilead, ViiV, and MSD. MVC has reports payment or honoraria for presentations, manuscript writing, or educational events from MSD, ViiV, Gilead, and Janssen-Cilag, DM received grants and fees for the speaker bureau, and CME activities from ViiV Healthcare, Merck & Co. Inc., Gilead Science Inc., and Viatris Inc.; fees for advisory boards from Johnson & Johnson and Gilead Science Inc., and non-financial educational support from Gilead Sciences Inc. and ViiV Healthcare., CG has received personal fees from MSD, ViiV, Gilead, and Janseen Cilag, outside the submitted work. S. A. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Pfizer, and support for attending meetings and/or travel from Pfizer and MSD. Andrea Gori reports grants or contracts from ViiV, Bristol-Myers Squibb, and Gilead; consulting fees from ViiV Healthcare, Gilead, Janssen-Cilag, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, and Novartis; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ViiV Healthcare, Gilead, Janssen-Cilag, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, and Novartis; support for attending meetings and/or travel from ViiVHealthcare, Gilead, Janssen-Cilag, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, and Novartis. GC, SR, FS, GP, MLC, CF, and ALR have nothing to declare.
Figures

References
-
- Sax P.E., Pozniak A., Montes M.L., Koenig E., DeJesus E., Stellbrink H.J., et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with Emtricitabine and Tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–2082. doi: 10.1016/S0140-6736(17)32340-1. - DOI - PubMed
-
- Sax P.E., Rockstroh J.K., Luetkemeyer A.F., Yazdanpanah Y., Ward D., Trottier B., et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis. 2021;73:e485–e493. doi: 10.1093/cid/ciaa988. - DOI - PMC - PubMed
-
- Gallant J., Lazzarin A., Mills A., Orkin C., Podzamczer D., Tebas P., et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–2072. doi: 10.1016/S0140-6736(17)32299-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

