Protein to biomaterials: Unraveling the antiviral and proangiogenic activities of Ac-Tβ1-17 peptide, a thymosin β4 metabolite, and its implications in peptide-scaffold preparation
- PMID: 40177110
- PMCID: PMC11964602
- DOI: 10.1016/j.bioactmat.2025.02.008
Protein to biomaterials: Unraveling the antiviral and proangiogenic activities of Ac-Tβ1-17 peptide, a thymosin β4 metabolite, and its implications in peptide-scaffold preparation
Abstract
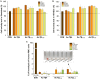
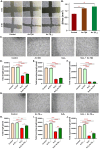
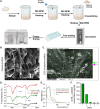
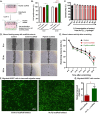
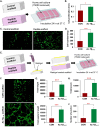
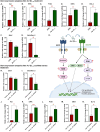
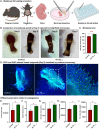
Peptide metabolites are emerging biomolecules with numerous possibilities in biomaterial-based regenerative medicine due to their inherent bioactivities. These small, naturally occurring compounds are intermediates or byproducts of larger proteins and peptides, and they can have profound effects, such as antiviral therapeutics, proangiogenic agents, and regenerative medicinal applications. This study is among the first to focus on using thymosin β4 protein-derived metabolites to pioneer novel applications for peptide metabolites in biomaterials. This study found that the novel peptide metabolite acetyl-thymosin β4 (amino acid 1-17) (Ac-Tβ1-17) exhibited significant protease inhibition activity against SARS-CoV-2, surpassing its precursor protein. Additionally, Ac-Tβ1-17 demonstrated beneficial effects, such as cell proliferation, wound healing, and scavenging of reactive oxygen species (ROS) in human umbilical vein endothelial cells (HUVEC). Integrating Ac-Tβ1-17 into a peptide-based scaffold facilitated cell growth and angiogenesis inside the scaffold and through gradual release into the surrounding environment. The Ac-Tβ1-17 peptide treatment induced significant biochemical responses in HUVEC, increasing Akt, ERK, PI3K, MEK, and Bcl-2 gene expression and proangiogenic proteins. Ac-Tβ1-17 peptide treatment showed similar results in ex vivo by enhancing mouse fetal metatarsal growth and angiogenesis. These findings highlight the potential of natural protein metabolites to generate biologically active peptides, offering a novel strategy for enhancing biomaterial compatibility. This approach holds promise for developing therapeutic biomaterials using peptide metabolites, presenting exciting prospects for future research and applications.
Keywords: Ac-Tβ1-17 peptide; Antiviral peptide; Peptide metabolites; Peptide-based biomaterial; Regenerative medicine; Thymosin β4.
© 2025 The Authors.
Conflict of interest statement
Hyung-Seop Han is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. The authors declare the following personal relationships which may be considered as potential competing interests: Hyung-Seop Han is currently employed by Elecell Corporation.
Figures





Similar articles
-
Detection and quantification of the metabolite Ac-Tβ1-14 in in vitro experiments and urine of rats treated with Ac-Tβ4: A potential biomarker of Ac-Tβ4 for doping tests.Drug Test Anal. 2023 Nov-Dec;15(11-12):1454-1467. doi: 10.1002/dta.3552. Epub 2023 Jul 28. Drug Test Anal. 2023. PMID: 37515313
-
Therapeutic potential of thymosin-beta4 and its derivative N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) in cardiac healing after infarction.Am J Cardiovasc Drugs. 2006;6(5):305-11. doi: 10.2165/00129784-200606050-00003. Am J Cardiovasc Drugs. 2006. PMID: 17083265 Review.
-
Thymosin beta4 enhances endothelial cell differentiation and angiogenesis.Angiogenesis. 1999;3(2):125-35. doi: 10.1023/a:1009041911493. Angiogenesis. 1999. PMID: 14517430
-
The actin binding site on thymosin beta4 promotes angiogenesis.FASEB J. 2003 Nov;17(14):2103-5. doi: 10.1096/fj.03-0121fje. Epub 2003 Sep 18. FASEB J. 2003. PMID: 14500546
-
Thymosin β as an Actin-binding Protein with a Variety of Functions.Adv Clin Exp Med. 2016 Nov-Dec;25(6):1331-1336. doi: 10.17219/acem/32026. Adv Clin Exp Med. 2016. PMID: 28028989 Review.
References
-
- Hamley I.W. Small bioactive peptides for biomaterials Design and therapeutics. Chem Rev. 2017;117(24):14015–14041. - PubMed
-
- Lachowicz J.I., Pichiri G., Piludu M., Fais S., Orru G., Congiu T., Piras M., Faa G., Fanni D., Dalla Torre G., Lopez X., Chandra K., Szczepski K., Jaremko L., Ghosh M., Emwas A.H., Castagnola M., Jaremko M., Hannappel E., Coni P. Thymosin beta4 is an endogenous Iron Chelator and molecular Switcher of Ferroptosis. Int. J. Mol. Sci. 2022;23(1) - PMC - PubMed
-
- Sosne G., Qiu P., Goldstein A.L., Wheater M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010;24(7):2144–2151. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

