A randomized phase II/III trial of rosuvastatin with neoadjuvant chemo-radiation in patients with locally advanced rectal cancer
- PMID: 40177244
- PMCID: PMC11961435
- DOI: 10.3389/fonc.2025.1450602
A randomized phase II/III trial of rosuvastatin with neoadjuvant chemo-radiation in patients with locally advanced rectal cancer
Abstract
Aim: Statins have been shown to improve the possibility of a pathological complete response (pCR) in patients with locally advanced rectal cancer when given in combination with neo-adjuvant chemo-radiation (NACTRT) in observational studies. The primary objective of this phase II randomized controlled trial (RCT) is to determine the impact of rosuvastatin in improving pCR rates in patients with locally advanced rectal cancer who are undergoing NACTRT. The secondary objectives are to compare adverse events, postoperative morbidity and mortality, disease-free survival (DFS), and overall survival in the two arms and to identify potential prognostic and predictive factors determining outcomes. If the study is positive, we plan to proceed to a phase III RCT with 3-year DFS as the primary endpoint.
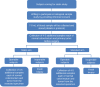
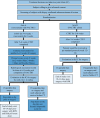
Methods: This is a prospective, randomized, open-label phase II/III study. The phase II study has a sample size of 316 patients (158 in each arm) to be accrued over 3 years to have 288 evaluable patients. The standard arm will receive NACTRT while the intervention group will receive 20 mg rosuvastatin orally once daily along with NACTRT for 6 weeks followed by rosuvastatin alone for 6-10 weeks until surgery. All patients will be reviewed after repeat imaging by a multidisciplinary tumor board at 12-16 weeks after starting NACTRT and operable patients will be planned for surgery. The pathological response rate, tumor regression grade (TRG), and post-surgical complications will be recorded.
Conclusion: The addition of rosuvastatin to NACTRT may improve the oncological outcomes by increasing the likelihood of pCR in patients with locally advanced rectal cancer undergoing NACTRT. This would be a low-cost, low-risk intervention that could potentially lead to the refinement of strategies, such as "watch and wait", in a select subgroup of patients.
Clinical trial registration: Clinical Trials Registry of India, identifier CTRI/2018/11/016459.
Keywords: drug repurposing; neoadjuvant chemoradiation; pathological complete response; rectal cancer; rosuvastatin.
Copyright © 2025 Patil, Saklani, Kumar, De’Souza, Krishnatry, Khanvilkar, Kazi, Engineer, Ostwal, Ramaswamy, Bal, Ranganathan, Gupta and Galande.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Fact Sheets by Population – CRC India ASRs . Available online at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (Accessed May 10, 2024).
-
- van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo pre-operative chemoradiotherapy for locally advanced rectal cancer re-staged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. (2013) 269:101–12. doi: 10.1148/radiol.13122833 - DOI - PubMed
LinkOut - more resources
Full Text Sources