Light and protonation-controlled complex formation between sulfate ions and a stiff-stilbene based bis(cyclopeptide)
- PMID: 40177314
- PMCID: PMC11959293
- DOI: 10.1039/d5sc00766f
Light and protonation-controlled complex formation between sulfate ions and a stiff-stilbene based bis(cyclopeptide)
Abstract
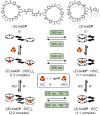
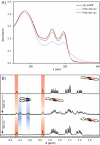
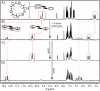
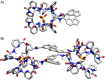
Anion-ligand coordination has been used to generate a number of supramolecular structures. Of particular interest is the transformation between different types of complexes using various stimuli. While there are multiple examples where this has been achieved with metal-ligand coordination complexes through incorporation of molecular photoswitches, the same has not yet been realized with anion-ligand coordination-driven assemblies. In this study, a sulfate-binding bis(cyclopeptide) with a photoswitchable stiff-stilbene linker is presented. Its (E)- and (Z)-isomers, and the different degrees of protonation of the anion (HSO4 - vs. SO4 2-), give rise to different assembly states. The accessible products have 1 : 1, 1 : 2 and 2 : 2 host-guest stoichiometries and can be interconverted by light irradiation and acid/base addition, resulting in a highly controllable responsive system that demonstrates the potential of sulfate coordination-driven supramolecular assembly.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Anion-Coordination-Driven Assembly.Acc Chem Res. 2022 Nov 15;55(22):3218-3229. doi: 10.1021/acs.accounts.2c00435. Epub 2022 Nov 4. Acc Chem Res. 2022. PMID: 36331808
-
Modulation of a Supramolecular Figure-of-Eight Strip Based on a Photoswitchable Stiff-Stilbene.Chemistry. 2020 Jun 23;26(35):7783-7787. doi: 10.1002/chem.202002051. Epub 2020 Jun 3. Chemistry. 2020. PMID: 32343010 Free PMC article.
-
A Photoswitchable Macrocycle Controls Anion-Templated Pseudorotaxane Formation and Axle Relocalization.Angew Chem Int Ed Engl. 2024 Jan 22;63(4):e202316628. doi: 10.1002/anie.202316628. Epub 2023 Dec 18. Angew Chem Int Ed Engl. 2024. PMID: 38059917
-
Anion Recognition in Aqueous Media by Cyclopeptides and Other Synthetic Receptors.Acc Chem Res. 2017 Nov 21;50(11):2870-2878. doi: 10.1021/acs.accounts.7b00458. Epub 2017 Nov 10. Acc Chem Res. 2017. PMID: 29125287 Review.
-
Photoresponsive Supramolecular Cages and Macrocycles.Chempluschem. 2023 Dec;88(12):e202300353. doi: 10.1002/cplu.202300353. Epub 2023 Sep 21. Chempluschem. 2023. PMID: 37638597 Review.
References
-
- Yoshizawa M. Klosterman J. K. Fujita M. Angew. Chem., Int. Ed. 2009;48:3418–3438. doi: 10.1002/anie.200805340. - DOI - PubMed
- Chakrabarty R. Mukherjee P. S. Stang P. J. Chem. Rev. 2011;111:6810–6918. - PMC - PubMed
- Smulders M. M. J. Riddell I. A. Browne C. Nitschke J. R. Chem. Soc. Rev. 2013;42:1728–1754. - PubMed
-
- Wezenberg S. J. Chem. Lett. 2020;49:609–615.
- Benchimol E. Tessarolo J. Clever G. H. Nat. Chem. 2024;16:13–21. - PubMed
-
- Sun S.-S. Anspach J. A. Lees A. J. Inorg. Chem. 2002;41:1862–1869. doi: 10.1021/ic010998e. - DOI - PubMed
- Chen S. Chen L.-J. Yang H.-B. Tian H. Zhu W. J. Am. Chem. Soc. 2012;134:13596–13599. - PubMed
- Han M. Michel R. He B. Chen Y.-S. Stalke D. John M. Clever G. H. Angew. Chem., Int. Ed. 2013;52:1319–1323. - PubMed
- Yan X. Xu J.-F. Cook T. R. Huang F. Yang Q.-Z. Tung C.-H. Stang P. J. Proc. Natl. Acad. Sci. U. S. A. 2014;111:8717–8722. - PMC - PubMed
- Han M. Luo Y. Damaschke B. Gómez L. Ribas X. Jose A. Peretzki P. Seibt M. Clever G. H. Angew. Chem., Int. Ed. 2016;55:445–449. - PubMed
- Stuckhardt C. Roke D. Danowski W. Otten E. Wezenberg S. J. Feringa B. L. Beilstein J. Org. Chem. 2019;15:2767–2773. - PMC - PubMed
- Kennedy A. D. W. DiNardi R. G. Fillbrook L. L. Donald W. A. Beves J. E. Chem.–Eur. J. 2022;28:e202104461. - PMC - PubMed
- DiNardi R. G. Douglas A. O. Tian R. Price J. R. Tajik M. Donald W. A. Beves J. E. Angew. Chem., Int. Ed. 2022;61:e202205701. - PMC - PubMed
- Hugenbusch D. Lehr M. von Glasenapp J.-S. McConnell A. J. Herges R. Angew. Chem., Int. Ed. 2023;62:e202212571. - PMC - PubMed
- Zhu J. Chen X. Jin X. Wang Q. Chin. Chem. Lett. 2023;34:108002. doi: 10.1016/j.cclet.2022.108002. - DOI
- DiNardi R. G. Rasheed S. Capomolla S. S. Him Chack M. Middleton I. A. Macreadie L. K. Violi J. P. Donald W. A. Lusby P. J. Beves J. E. J. Am. Chem. Soc. 2024;146:21196–21202. doi: 10.1021/jacs.4c04846. - DOI - PMC - PubMed
-
-
For review articles, see:
- Busschaert N. Caltagirone C. Van Rossom W. Gale P. A. Chem., Rev. 2015;115:8038–8155. - PubMed
- Yang D. Zhao J. Yang X.-J. Wu B. Org. Chem. Front. 2018;5:662–690.
- Zhao J. Yang D. Yang X.-J. Wu B. Coord. Chem. Rev. 2019;378:415–444.
- Boer S. A. Foyle E. M. Thomas C. M. White N. G. Chem. Soc. Rev. 2019;48:2596–2614. - PubMed
- Zhang W. Zhao J. Yang D. ChemPlusChem. 2022;87:e202200294. - PubMed
-
LinkOut - more resources
Full Text Sources
Miscellaneous

