Iron ore pellets based-Ag2O nanoparticles as efficient Bi-functional heterogeneous catalyst for the synthesis tetrahydrobenzo[α]xanthens in green media
- PMID: 40177351
- PMCID: PMC11962902
- DOI: 10.3389/fchem.2025.1413080
Iron ore pellets based-Ag2O nanoparticles as efficient Bi-functional heterogeneous catalyst for the synthesis tetrahydrobenzo[α]xanthens in green media
Abstract
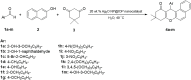
Through the use of a microwave, iron ore pellets (IOP)-based Ag2O nanoparticles were successfully synthesized. They were then characterized by means of a vibrating sample magnetometer (VSM), Brunauer-Emmett-Teller (BET) surface area analysis, energy-dispersive X-ray (EDX) analysis, powder X-ray diffraction (XRD) analysis, EDX elemental mapping, and field emission scanning electron microscopy (FESEM). High quantities of tetrahydrobenzo[a]xanthen derivatives were obtained in a brief amount of time by the newly prepared nanocomposite, known as Ag2O NP@IOP, in a one-pot, three-component reaction involving different aryl aldehydes, naphthol, and dimedone. There is no appreciable loss of catalytic activity when the catalyst is recycled and utilized several times, and it can be easily retrieved using an external magnet. The reason for functionality of designed hybrid catalyst can be related to textural properties such as desirable specific surface area and significant porosity as well as the structural nature of the Ag2O NP@IOP catalyst.
Keywords: Ag2O NP@IOP; benzoxanthens; green synthesis; iron ore pellets; multi-component reaction; reusable catalyst.
Copyright © 2025 Faryabi, Sheikhhosseini and Yahyazadehfar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures













References
-
- Abad S. S. S., Mirjalili B. B. F., Bamoniri A. (2023). Fe3O4@ nano-walnut shell/BIII as a new natural based catalyst for synthesis of tetrahydrobenzo [a] xanthene-11-one derivatives. Polycycl. Aromat. Compd. 43, 7979–7991. 10.1080/10406638.2022.2144907 - DOI
-
- Abel O. T., Ogundana A. K. (2014). Preliminary quality and potential assessment of groundwater in the basement complex terrain of ijero-ekiti, southwestern Nigeria. Int. J. Innov. Res. Sci. Stud. 3, 15100–15107. 10.15680/IJIRSET.2014.0308007 - DOI
-
- Agrwal A., Kumar V., Kasana V. (2021). Preparation and application of highly efficient and reusable TBAPIL@ Si(CH2)3@ nano-silica-based nano-catalyst for preparation of benzoxanthene derivatives. J. Iran. Chem. Soc. 18, 2583–2595. 10.1007/s13738-021-02211-1 - DOI
-
- Bamoniri A., Yaghmaeiyan N., Yaghmaeiyan N. (2022). Synthesis of 9, 9-dimethyl-12-(aryl)-8, 9, 10, 12-tetrahydrobenzo [a] xanthene-11-ones by modified kaolinite nanoclay as an efficient and reusable heterogeneous catalyst via a green protocol. Indian J. Chem. 61, 599–606. 10.56042/ijc.v61i6.64203 - DOI
-
- Bedi P., Behera A. K., Alanazi A. K., Roy M., Das S., Shukla R., et al. (2023). Benzoxanthones derivatives: insight into oxalic acid catalyzed synthesis, crystallographic analysis and potential application in dye-sensitized solar cell. Chem. Sci. J. 135, 34–47. 10.1007/s12039-023-02151-8 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

