Exploring Scent Distinction with Polymer Brush Arrays
- PMID: 40177398
- PMCID: PMC11959526
- DOI: 10.1021/acsapm.5c00066
Exploring Scent Distinction with Polymer Brush Arrays
Abstract
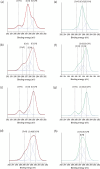
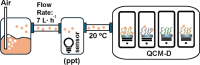
The ability to distinguish scents, volatile organic compounds (VOCs), and their mixtures is critical in agriculture, food safety, and public health. This study introduces a proof-of-concept approach for VOC and scent distinction, leveraging polymer brush arrays with diverse chemical compositions designed to interact with various VOCs and scents. When VOCs or scents are exposed to the brush array, they produce distinct mass absorption patterns for different polymer brushes, effectively creating "fingerprints". Scents can be recognized without having to know the absorption of their individual components. This allows for a scent distinction technique, mimicking scent recognition within a mammalian olfactory system. To demonstrate the scent distinction, we synthesized different polymer brushes, zwitterionic, hydrophobic, and hydrophilic, using surface-initiated photoinduced electron transfer-reversible addition-fragmentation chain-transfer polymerization with eosin Y and triethanolamine as catalysts. The polymer brushes were then exposed to vapors of different single-compound VOCs and complex scents consisting of many VOCs, such as the water-ethanol mixture, rosemary oil, lavender oil, and whiskey scents. Quartz crystal microbalance measurements with dissipation monitoring (QCM-D) show a clear difference in brush absorption for these diverse VOC vapors such that distinct fingerprints can be identified. Our proof-of-concept study aims to pave the way for universal electronic nose sensors that distinguish scents by combining mass absorption patterns from polymer brush-coated surfaces.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- de March C. A.; Ryu S.; Sicard G.; Moon C.; Golebiowski J. Structure–odour relationships reviewed in the postgenomic era. Flavour Fragr. J. 2015, 30 (5), 342–361. 10.1002/ffj.3249. - DOI
-
- Williams J.; Koppmann R. . In Volatile Organic Compounds in the Atmosphere; Wiley, 2007; pp 1–32.
-
- Rodriguez J. L.; Almirall J. R. Continuous vapor sampling of volatile organic compounds associated with explosives using capillary microextraction of volatiles (CMV) coupled to a portable GC–MS. Forensic Chem. 2021, 26, 100380.10.1016/j.forc.2021.100380. - DOI
-
- Muto A.; Müller C. T.; Bruno L.; McGregor L.; Ferrante A.; Chiappetta A. A. C.; Bitonti M. B.; Rogers H. J.; Spadafora N. D. Fruit volatilome profiling through GC × GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10 (1), 18333.10.1038/s41598-020-75322-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

