Analysis of DNA from brain tissue on stereo-EEG electrodes reveals mosaic epilepsy-related variants
- PMID: 40177531
- PMCID: PMC11961356
- DOI: 10.1093/braincomms/fcaf113
Analysis of DNA from brain tissue on stereo-EEG electrodes reveals mosaic epilepsy-related variants
Erratum in
-
Correction to: Analysis of DNA from brain tissue on stereo-EEG electrodes reveals mosaic epilepsy-related variants.Brain Commun. 2025 Apr 29;7(2):fcaf160. doi: 10.1093/braincomms/fcaf160. eCollection 2025. Brain Commun. 2025. PMID: 40303601 Free PMC article.
Abstract
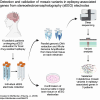
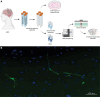
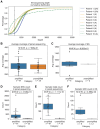
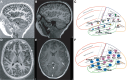
Somatic mosaic variants contribute to focal epilepsy, with variants often present only in brain tissue and not in blood or other samples typically assayed for genetic testing. Thus, genetic analysis for mosaic variants in focal epilepsy has been limited to patients with drug-resistant epilepsy who undergo surgical resection and have resected brain tissue samples available. Stereo-EEG (sEEG) has become part of the evaluation for many patients with focal drug-resistant epilepsy, and sEEG electrodes provide a potential source of small amounts of brain-derived DNA. We aimed to identify, validate, and assess the distribution of deleterious mosaic variants in epilepsy-associated genes in DNA extracted from trace brain tissue on individual sEEG electrodes. We enrolled a prospective cohort of 10 paediatric patients with drug-resistant epilepsy who had sEEG electrodes implanted for invasive monitoring. We extracted unamplified DNA and in parallel performed whole-genome amplification from trace brain tissue on each sEEG electrode. We also extracted DNA from resected brain tissue and blood/saliva samples where available. We performed deep sequencing (panel and exome) and analysis for candidate germline and mosaic variants. We validated candidate mosaic variants and assessed the variant allele fraction in amplified and unamplified electrode-derived DNA and across electrodes. We extracted unamplified DNA and performed whole-genome amplification from >150 individual electrodes from 10 individuals. Immunohistochemistry confirmed the presence of neurons in the brain tissue on electrodes. Deep sequencing and analysis demonstrated similar depth of coverage between amplified and unamplified DNA samples but significantly more potential mosaic variants in amplified samples. We validated four deleterious mosaic variants in epilepsy-associated genes in electrode-derived DNA in three patients who underwent laser ablation and did not have resected brain tissue samples available. Three of the four variants were detected in both amplified and unamplified electrode-derived DNA, with higher variant allele fraction observed in DNA from electrodes in closest proximity to the electrical seizure focus in one case. We demonstrate that mosaic variants can be identified and validated from DNA extracted from trace brain tissue on individual sEEG electrodes in patients with drug-resistant focal epilepsy, from both unamplified and amplified electrode-derived DNA. Our findings support a relationship between the extent of regional genetic abnormality and electrophysiology and suggest that with further optimization, this minimally invasive diagnostic approach holds promise for advancing precision medicine for patients with drug-resistant epilepsy as part of the surgical evaluation.
Keywords: drug-resistant epilepsy; epilepsy genetics; epilepsy surgery; somatic mosaicism; stereo-EEG.
Published by Oxford University Press on behalf of the Guarantors of Brain 2025.
Conflict of interest statement
The authors report no competing interests.
Figures
Update of
-
Analysis of DNA from brain tissue on stereo-EEG electrodes reveals mosaic epilepsy-related variants.medRxiv [Preprint]. 2024 Jul 22:2024.07.21.24310779. doi: 10.1101/2024.07.21.24310779. medRxiv. 2024. Update in: Brain Commun. 2025 Mar 17;7(2):fcaf113. doi: 10.1093/braincomms/fcaf113. PMID: 39108522 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
