Exploring recent advances in signaling pathways and hallmarks of uveal melanoma: a comprehensive review
- PMID: 40177537
- PMCID: PMC11964777
- DOI: 10.37349/etat.2025.1002306
Exploring recent advances in signaling pathways and hallmarks of uveal melanoma: a comprehensive review
Abstract
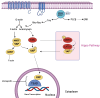
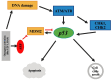
The purpose of this review was to provide a comprehensive review of the latest insights on the pathogenesis of uveal melanoma (UM) and its intracellular pathways. This article covers the epidemiology of UM, racial predispositions, cytogenetic and chromosomal alterations, gene mutations, key defective pathways, and their underlying mechanisms, as well as the application of hallmarks of cancer to UM. A key knowledge gap remains in identifying the most effective targeted therapy and determining the central pathway linking multiple signaling networks. UM is a malignant tumor arising from uveal melanocytes, predominantly affecting the choroid, with both genetic and epigenetic contributors. Key cytogenetic alterations include monosomy 3, chromosome 6p gain, chromosome 1p loss, and chromosome 8q gain. The most important UM-related signaling pathways are RAS/MAPK, PI3K/Akt/mTOR, Hippo-YAP, retinoblastoma (Rb), and p53 pathways. In the RAS/MAPK pathway, GNAQ/GNA11 mutations occur which account for more than 80% of UM cases. The PI3K/Akt/mTOR pathway promotes cyclin D1 overexpression and MDM2 upregulation, leading to p53 pathway inhibition. GNAQ/GNA11 mutations activate YAP via the Trio-RhoGTPase/RhoA/Rac1 signaling circuit in the Hippo-YAP pathway. Rb pathway dysregulation results from cyclin D1 overexpression or cyclin-dependent kinase inhibitor (CDKI) inactivation. In the p53 pathway, UM is characterized by p53 mutations, MDM2 overexpression, and Bcl-2 deregulation. Eventually, the ARF-MDM2 axis serves as a critical link between the RAS and p53 pathways. Hallmarks of cancer, such as evasion of growth suppression and self-sufficiency in growth signals, are also evident in UM. Genetic and epigenetic alterations, including NSB1, MDM2 and CCND1 amplification, and BAP1 mutations, play pivotal roles in UM pathobiology. Thus, UM exhibits a multifactorial pathology. By consolidating key mechanisms underlying UM pathogenesis, this review provides a comprehensive perspective on the involved pathways, offering insights that may facilitate the development of effective therapeutic strategies.
Keywords: Akt pathway; Hippo pathway; Uveal melanoma; hallmarks of cancer; p53 pathway; rat sarcoma virus (RAS) pathway; retinoblastoma (Rb) pathway.
© The Author(s) 2025.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
