Celastrol directly targets LRP1 to inhibit fibroblast-macrophage crosstalk and ameliorates psoriasis progression
- PMID: 40177548
- PMCID: PMC11959968
- DOI: 10.1016/j.apsb.2024.12.041
Celastrol directly targets LRP1 to inhibit fibroblast-macrophage crosstalk and ameliorates psoriasis progression
Abstract
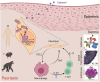
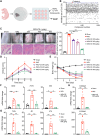
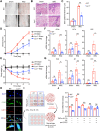
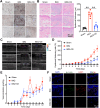
Psoriasis is an incurable chronic inflammatory disease that requires new interventions. Here, we found that fibroblasts exacerbate psoriasis progression by promoting macrophage recruitment via CCL2 secretion by single-cell multi-omics analysis. The natural small molecule celastrol was screened to interfere with the secretion of CCL2 by fibroblasts and improve the psoriasis-like symptoms in both murine and cynomolgus monkey models. Mechanistically, celastrol directly bound to the low-density lipoprotein receptor-related protein 1 (LRP1) β-chain and abolished its binding to the transcription factor c-Jun in the nucleus, which in turn inhibited CCL2 production by skin fibroblasts, blocked fibroblast-macrophage crosstalk, and ameliorated psoriasis progression. Notably, fibroblast-specific LRP1 knockout mice exhibited a significant reduction in psoriasis like inflammation. Taken together, from clinical samples and combined with various mouse models, we revealed the pathogenesis of psoriasis from the perspective of fibroblast-macrophage crosstalk, and provided a foundation for LRP1 as a novel potential target for psoriasis treatment.
Keywords: CCL2; Celastrol; Drug target; Fibroblast; LRP1; Macrophage; Psoriasis; c-Jun.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Boehncke W.H., Schön M.P. Psoriasis. Lancet. 2015;386:983–994. - PubMed
-
- Griffiths C.E., Armstrong A.W., Gudjonsson J.E., Barker J.N.W.N. Psoriasis. Lancet. 2021;397:1301–1315. - PubMed
-
- Rachakonda T.D., Schupp C.W., Armstrong A.W. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. - PubMed
-
- Duvallet E., Semerano L., Assier E., Falgarone G., Boissier M.C. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med. 2011;43:503–511. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

