Intestinal stearoyl-coenzyme A desaturase-inhibition improves obesity-associated metabolic disorders
- PMID: 40177566
- PMCID: PMC11959918
- DOI: 10.1016/j.apsb.2024.11.022
Intestinal stearoyl-coenzyme A desaturase-inhibition improves obesity-associated metabolic disorders
Abstract
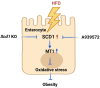
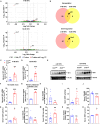
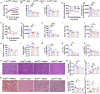
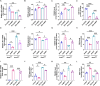
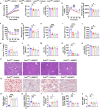
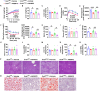
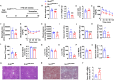
Stearoyl-coenzyme A desaturase 1 (SCD1) catalyzes the rate-limiting step of de novo lipogenesis and modulates lipid homeostasis. Although numerous SCD1 inhibitors were tested for treating metabolic disorders both in preclinical and clinic studies, the tissue-specific roles of SCD1 in modulating obesity-associated metabolic disorders and determining the pharmacological effect of chemical SCD1 inhibition remain unclear. Here a novel role for intestinal SCD1 in obesity-associated metabolic disorders was uncovered. Intestinal SCD1 was found to be induced during obesity progression both in humans and mice. Intestine-specific, but not liver-specific, SCD1 deficiency reduced obesity and hepatic steatosis. A939572, an SCD1-specific inhibitor, ameliorated obesity and hepatic steatosis dependent on intestinal, but not hepatic, SCD1. Mechanistically, intestinal SCD1 deficiency impeded obesity-induced oxidative stress through its novel function of inducing metallothionein 1 in intestinal epithelial cells. These results suggest that intestinal SCD1 could be a viable target that underlies the pharmacological effect of chemical SCD1 inhibition in the treatment of obesity-associated metabolic disorders.
Keywords: High-fat diet; Intestinal epithelium; MT1; Metabolic disorders; Obesity; Oxidative stress; SCD1; Steatosis.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures
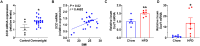

References
-
- Bray G.A., Kim K.K., Wilding J.P.H. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–723. - PubMed
-
- Haslam D.W., James W.P. Obesity. Lancet. 2005;366:1197–1209. - PubMed
-
- Nogueiras R., Nauck M.A., Tschop M.H. Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat Metab. 2023;5:933–944. - PubMed
LinkOut - more resources
Full Text Sources

