Deubiquitinase JOSD2 alleviates colitis by inhibiting inflammation via deubiquitination of IMPDH2 in macrophages
- PMID: 40177575
- PMCID: PMC11959961
- DOI: 10.1016/j.apsb.2024.12.012
Deubiquitinase JOSD2 alleviates colitis by inhibiting inflammation via deubiquitination of IMPDH2 in macrophages
Abstract
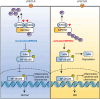
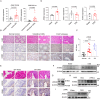
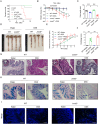
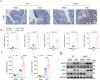
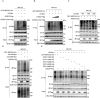
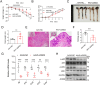
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, which increases the incidence of colorectal cancer (CRC). In the pathophysiology of IBD, ubiquitination/deubiquitination plays a critical regulatory function. Josephin domain containing 2 (JOSD2), a deubiquitinating enzyme, controls cell proliferation and carcinogenesis. However, its role in IBD remains unknown. Colitis mice model developed by dextran sodium sulfate (DSS) or colon tissues from individuals with ulcerative colitis and Crohn's disease showed a significant upregulation of JOSD2 expression in the macrophages. JOSD2 deficiency exacerbated the phenotypes of DSS-induced colitis by enhancing colon inflammation. DSS-challenged mice with myeloid-specific JOSD2 deletion developed severe colitis after bone marrow transplantation. Mechanistically, JOSD2 binds to the C-terminal of inosine-5'-monophosphate dehydrogenase 2 (IMPDH2) and preferentially cleaves K63-linked polyubiquitin chains at the K134 site, suppressing IMPDH2 activity and preventing activation of nuclear factor kappa B (NF-κB) and inflammation in macrophages. It was also shown that JOSD2 knockout significantly exacerbated increased azoxymethane (AOM)/DSS-induced CRC, and AAV6-mediated JOSD2 overexpression in macrophages prevented the development of colitis in mice. These outcomes reveal a novel role for JOSD2 in colitis through deubiquitinating IMPDH2, suggesting that targeting JOSD2 is a potential strategy for treating IBD.
Keywords: Colitis; Deubiquitinase; IMPDH2; Inflammation; Inflammatory bowel disease; JOSD2; Macrophage; Nuclear factor kappa B.
© 2025 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Siegel R.L., Miller K.D., Sauer A.G., Fedewa S.A., Butterly L.F., Anderson J.C., et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. - PubMed
-
- Faye A.S., Holmer A.K., Axelrad J.E. Cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2022;51:649–666. - PubMed
-
- Parikh K., Antanaviciute A., Fawkner-Corbett D., Jagielowicz M., Aulicino A., Lagerholm C., et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49–55. - PubMed
LinkOut - more resources
Full Text Sources

