Unusual Rearrangement of a 1,8-Naphthalene Derivative
- PMID: 40177727
- PMCID: PMC12129256
- DOI: 10.1021/acs.joc.5c00021
Unusual Rearrangement of a 1,8-Naphthalene Derivative
Abstract
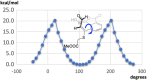
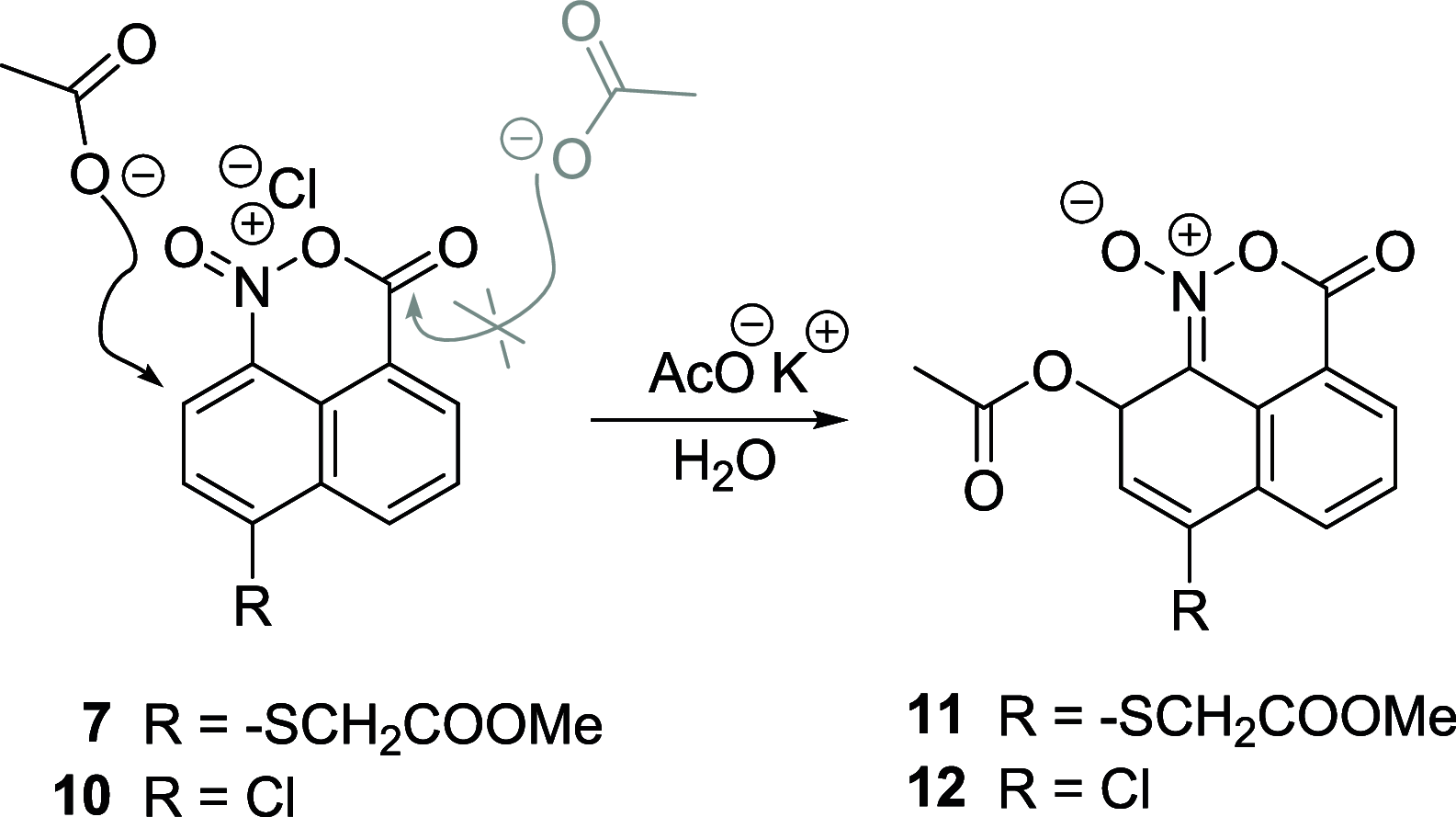
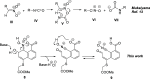
The steric strain between nitro and carboxylic acid groups in an 8-nitro-1-naphthoic acid derivative is able to unexpectedly disrupt the aromaticity of the naphthalene core under mild reaction conditions. The addition of H2O to the aromatic ring of a highly strained naphtho oxazinium intermediate induces the fragmentation of a Csp2-Csp2 bond, with a concomitant rearrangement to yield a conjugated aldehyde. Key intermediates have been characterized, and the X-ray structure of the derivative has been obtained. Density functional theory (DFT) studies were performed to confirm the proposed mechanism.
Figures

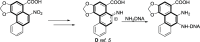


References
-
- Anderson J. E., Cooksey C. J.. peri-Interactions in Naphthalene Derivatives. Rotation in 1,8-Disubstituted Naphthalenes. J. Chem. Soc., Chem. Commun. 1975:942–943. doi: 10.1039/c39750000942. - DOI
-
- Schweizer W. B., Procter G., Kaftory M., Dünitz J. D.. Structural Studies of 1,8-Disubstituted Naphthalenes as Probes for Nucleophile-Electrophile Interactions. Helv. Chim. Acta. 1978;61:2783–2808. doi: 10.1002/hlca.19780610806. - DOI
-
- Bristow J. C., Leslie R., Wallis J. D.. Incipient Nucleophilic Attack on a Carbonyl Group Adjacent to a Stereogenic Centre in Peri Naphthalene Derivatives. Helv. Chim. Acta. 2023;106:e202300021. doi: 10.1002/hlca.202300021. - DOI
- Bristow J. C., Cliff S. V. A., Yang S., Wallis J. D.. Interaction, bond formation or reaction between a dimethylamino group and an adjacent alkene or aldehyde group in aromatic systems controlled by remote molecular constraints. CrystEngComm. 2021;23:4500–4512. doi: 10.1039/D1CE00377A. - DOI
- Bristow J. C., Naftalin I., Cliff S. V. A., Yang S., Carravetta M., Heinmaa I., Stern R., Wallis J. D.. Modelling of an aza-Michael reaction from crystalline naphthalene derivatives containing peri–peri interactions: very long N–C bonds? CrystEngComm. 2020;22:6783–6795. doi: 10.1039/D0CE01137A. - DOI
- Saritemur G., Miralles L. N., Husson D., Pitak M. B., Coles S. J., Wallis J. D.. Two modes of peri-interaction between an aldehyde group and a carboxylate anion in naphthalaldehydate salts. CrystEngComm. 2016;18:948–961. doi: 10.1039/C5CE02282G. - DOI
- Wannebroucq A., Jarmyn A. P., Pitak M. B., Coles S. J., Wallis J. D.. Reactions and Interactions Between Peri Groups in 1- Dimethylamino-Naphthalene Salts: An Example of a “Through Space” Amide. Pure Appl. Chem. 2016;88:317–331. doi: 10.1515/pac-2015-1103. - DOI
- Mercadal N., Day S. P., Jarmyn A., Pitak M. B., Coles S. J., Wilson C., Rees G. J., Hanna J. V., Wallis J. D.. O- vs. N-protonation of 1-dimethylaminonaphthalene-8-ketones: formation of a peri N–C bond or a hydrogen bond to the pi-electron density of a carbonyl group. CrystEngComm. 2014;16:8363–8837. doi: 10.1039/C4CE00981A. - DOI
- Lari A., Pitak M. B., Coles S. J., Rees G. J., Day S. P., Smith M. E., Hanna V. J., Wallis J. D.. Models for incomplete nucleophilic attack on a protonated carbonyl group and electron-deficient alkenes: salts and zwitterions from 1-dimethylamino-naphthalene-8-carbaldehyde. Org. Biomol. Chem. 2012;10:7763–7779. doi: 10.1039/c2ob25929j. - DOI - PubMed
-
- Priestap H. A., Barbieri M. A., Johnson F.. Aristoxazole Analogues. Conversion of 8-Nitro-1-naphthoic Acid to 2-Methylnaphtho[1,2-d]oxazole-9-carboxylic Acid: Comments on the Chemical Mechanism of Formation of DNA Adducts by the Aristolochic Acids. J. Nat. Prod. 2012;75:1414–1418. doi: 10.1021/np300137f. - DOI - PubMed
LinkOut - more resources
Full Text Sources

