Bone in Parathyroid Diseases Revisited: Evidence From Epidemiological, Surgical and New Drug Outcomes
- PMID: 40177730
- PMCID: PMC12259238
- DOI: 10.1210/endrev/bnaf010
Bone in Parathyroid Diseases Revisited: Evidence From Epidemiological, Surgical and New Drug Outcomes
Abstract
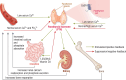
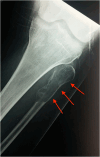
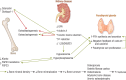
PTH-related disorders have a major impact on bone metabolism and skeletal properties because of the pivotal role of PTH in calcium and phosphate homeostasis and bone remodeling. Hyperparathyroidism is characterized by continuous exposure to excessive endogenous PTH, causing increased bone turnover in favor of bone resorption. Depending on the background of PTH overproduction, hyperparathyroidism is divided into primary, secondary, and tertiary hyperparathyroidism. The clinical presentation varies from deterioration of bone microarchitecture and decreased bone mineral density to profound bone involvement, such as osteitis fibrosa cystica and fragility fractures. Although successful parathyroidectomy represents the definitive treatment and may promote regression of most of the skeletal defects, the medical approach of calcimimetics and antiresorptive agents is a promising alternative in cases where parathyroidectomy is not feasible or unsuccessful. Hypoparathyroidism is the pathophysiological counterpart of hyperparathyroidism and also leads to disorders of bone metabolism and structure. Chronic PTH deprivation is associated with low bone remodeling and increased bone mineral density. The defective microarchitecture might affect bone strength and raise the risk for adverse skeletal events. Recombinant human PTH acts as a replacement therapy and is safe and efficient in restoring calcium/phosphate homeostasis and bone turnover. However, it is approved only for refractory cases, as conventional management with calcium and active vitamin D remains the first-line treatment. This article reviews the skeletal involvement in the most frequent parathyroid disorders, hyperparathyroidism and hypoparathyroidism, and rare familial disorders of PTH metabolism, as assessed by clinical, laboratory, and imaging parameters, and the effect of the available treatment strategies.
Keywords: bone; fracture; hyperparathyroidism; hypoparathyroidism; parathyroid disorders; pseudohypoparathyroidism.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Goltzman D. Physiology of parathyroid hormone. Endocrinol Metab Clin North Am. 2018;47(4):743‐758. - PubMed
-
- Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician. 2013;88(4):249‐257. - PubMed
-
- Fraser WD. Hyperparathyroidism. Lancet. 2009;374(9684):145‐158. - PubMed
-
- Tregear GW, Van Rietschoten J, Greene E, et al. Bovine parathyroid hormone: minimum chain length of synthetic peptide required for biological activity. Endocrinology. 1973;93(6):1349‐1353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

