Endothelium- and Fibroblast-Derived C-Type Natriuretic Peptide Prevents the Development and Progression of Aortic Aneurysm
- PMID: 40177775
- PMCID: PMC12188825
- DOI: 10.1161/ATVBAHA.124.322350
Endothelium- and Fibroblast-Derived C-Type Natriuretic Peptide Prevents the Development and Progression of Aortic Aneurysm
Abstract
Background: Thoracic (TAA) and abdominal (AAA) aortic aneurysm are life-threatening diseases characterized by dilation, inflammation, and structural weakness; development of pharmacological therapies is desperately needed. CNP (C-type natriuretic peptide) plays a key role in vascular homeostasis, mediating vasodilator, anti-inflammatory, and antiatherogenic actions. Since such processes drive AA, we determined the role of endogenous CNP in offsetting pathogenesis.
Methods: Tissue from patients with AA was analyzed to determine the consequences on CNP signaling. Ascending and suprarenal aortic diameters were assessed at baseline and following Ang II (angiotensin II; 1.44 mg/kg per day) infusion in wild-type, endothelium-restricted (ecCNP-/-), fibroblast-restricted (fbCNP-/-), global CNP (gbCNP-/-), or global NPR-C-/- mice infected with an adeno-associated virus expressing a proprotein convertase subtilisin/kexin type 9 gain-of-function mutation or backcrossed to an apoE-/- background. At 28 days, aortas were harvested for RT-qPCR (quantitative reverse transcription polymerase chain reaction) and histological analyses. CNP (0.2 mg/kg per day) was infused to rescue any adverse phenotype.
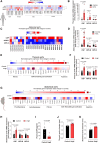
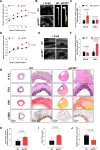
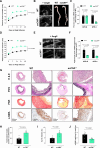
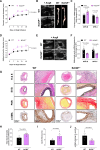
Results: Aneurysmal tissue from patients with TAA and AAA revealed that CNP and NPR-C (natriuretic peptide receptor-C) expression were overtly perturbed. ecCNP-/-, fbCNP-/-, and gbCNP-/- mice exhibited an aggravated phenotype compared to wild-type animals in both ascending and suprarenal aortas, exemplified by greater dilation, fibrosis, elastin degradation, and macrophage infiltration. CNP and NPR-C expression was also dysregulated in murine thoracic AA and abdominal AA, accompanied by increased accumulation of mRNA encoding markers of inflammation, extracellular matrix remodeling/calcification, fibrosis, and apoptosis. CNP also prevented activation of isolated macrophages and vascular smooth muscle cells. An essentially identical phenotype was observed in NPR-C-/- mice and while administration of CNP protected against disease severity in wild-type animals, this phenotypic rescue was not apparent in NPR-C-/- mice.
Conclusions: Endothelium- and fibroblast-derived CNP, via NPR-C activation, plays important roles in attenuating AA formation by preserving aortic structure and function. Therapeutic strategies aimed at mimicking CNP bioactivity hold potential to reduce the need for surgical intervention.
Keywords: aortic aneurysm; fibrosis; macrophages; natriuretic peptide, C-type; vascular remodeling.
Conflict of interest statement
A.J. Hobbs is a scientific advisory board member/consultant for Palatin Technologies, Inc, PharmaIN Corp, and Ascendis Pharma. S.A. LeMaire serves as a consultant for Cerus and has served as a principal investigator for clinical studies sponsored by Terumo Aortic and CytoSorbents. J.C. Tsui has received speaker’s honoraria from Bayer. The other authors have reported no conflicts.
Figures



Comment in
-
C-Type Natriuretic Peptide: Protecting the Aorta.Arterioscler Thromb Vasc Biol. 2025 Jul;45(7):1064-1066. doi: 10.1161/ATVBAHA.125.322802. Epub 2025 Jun 25. Arterioscler Thromb Vasc Biol. 2025. PMID: 40560989 No abstract available.
References
-
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7 - PMC - PubMed
-
- Raffort J, Lareyre F, Clement M, Hassen-Khodja R, Chinetti G, Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457–471. doi: 10.1038/nrcardio.2017.52 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

