Characterization of severe COL6-related dystrophy due to the recurrent variant COL6A1 c.930+189C>T
- PMID: 40177858
- PMCID: PMC12404708
- DOI: 10.1093/brain/awaf116
Characterization of severe COL6-related dystrophy due to the recurrent variant COL6A1 c.930+189C>T
Abstract
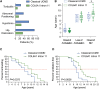
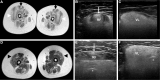
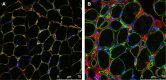
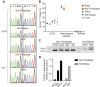
Collagen VI-related dystrophies manifest with a spectrum of clinical phenotypes, ranging from Ullrich congenital muscular dystrophy (UCMD), presenting with prominent congenital symptoms and characterized by progressive muscle weakness, joint contractures and respiratory insufficiency, to Bethlem muscular dystrophy, with milder symptoms typically recognized later and at times resembling a limb girdle muscular dystrophy, and intermediate phenotypes falling between UCMD and Bethlem muscular dystrophy. Despite clinical and muscle pathology features highly suggestive of collagen VI-related dystrophy, some patients had remained without an identified causative variant in COL6A1, COL6A2 or COL6A3. With combined muscle RNA sequencing and whole-genome sequencing, we uncovered a recurrent, de novo deep intronic variant in intron 11 of COL6A1 (c.930+189C>T) that leads to a dominantly acting in-frame pseudoexon insertion. We subsequently identified and have characterized an international cohort of 44 patients with this COL6A1 intron 11 causative variant, one of the most common recurrent causative variants in the collagen VI genes. Patients manifest a consistently severe phenotype characterized by a paucity of early symptoms followed by an accelerated progression to a severe form of UCMD, except for one patient with somatic mosaicism for this COL6A1 intron 11 variant who manifests a milder phenotype consistent with Bethlem muscular dystrophy. Partial amelioration of the disease phenotype in this individual provides a strong rationale for the development of our pseudoexon skipping therapy to successfully suppress the pseudoexon insertion, resulting in normal COL6A1 transcripts. We have previously shown that splice-modulating antisense oligomers applied in vitro effectively decreased the abundance of the mutant pseudoexon-containing COL6A1 transcripts to levels comparable to the in vivo scenario of the somatic mosaicism shown here, indicating that this therapeutic approach carries significant translational promise for ameliorating the severe form of UCMD caused by this common recurrent COL6A1 variant.
Keywords: COL6A1 c.930+189C>T; COL6A1 intron 11; collagen VI-related dystrophy; pseudoexon; splice-modulating; translational promise.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Bertini E, Pepe G. Collagen type VI and related disorders: Bethlem myopathy and Ullrich scleroatonic muscular dystrophy. Eur J Paediatr Neurol. 2002;6:193–198. - PubMed
-
- Brinas L, Richard P, Quijano-Roy S, et al. Early onset collagen VI myopathies: Genetic and clinical correlations. Ann Neurol. 2010;68:511–520. - PubMed

