Bioorthogonal Postlabeling Reveals Nuclear Localization of a Highly Cytotoxic Half-Sandwich Ir(III) Tetrazine Complex in Live Cells
- PMID: 40178169
- PMCID: PMC12177701
- DOI: 10.1002/cbic.202500090
Bioorthogonal Postlabeling Reveals Nuclear Localization of a Highly Cytotoxic Half-Sandwich Ir(III) Tetrazine Complex in Live Cells
Abstract
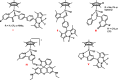
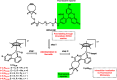
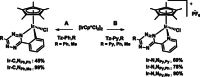
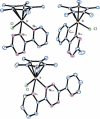
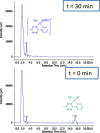
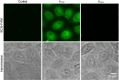
Intracellular imaging of anticancer metallodrugs often relies on prelabeling with organic fluorophores, which significantly affects their physicochemical properties and intracellular distribution. On the other hand, the reported postlabeling strategies based on click-chemistry reactions require cell fixation and permeabilization. Here, this study presents a postlabeling approach based on the catalyst-free, inverse electron-demand Diels-Alder reaction (iEDDA) between a strained fluorescein-tagged bicyclononyne derivative (BCN-FAM) and half-sandwich Ir(III) complexes containing bidentate ligands comprising a tetrazine (Tz-R,R') entity. Five half-sandwich Ir(III) complexes with formula [Cp*Ir(Tz-R,R')Cl]0/+ have been synthesized and fully characterized, including the X-ray crystal structures of three of the five derivatives. Investigations of their stability and their reactivity in aqueous solution and in a model iEDDA reaction reveal the strong influence of the tetrazine ligand structure on the chemical properties of the corresponding complexes. A highly cytotoxic metallodrug candidate (Ir-C,NPh,Me) is identified from biological studies, and chemical reactivity studies disclose an unusual preference for binding of methionine over cysteine. Postlabeling of Ir-C,NPh,Me in live HeLa cells highlights its preferential accumulation within the nucleus, suggesting its retention through covalent modifications of nuclear proteins in good agreement with other half-sandwich iridium(III) complexes.
Keywords: antitumor agents; cellular imaging; fluorescent probes; inverse electron‐demand Diels–Alder; iridium; solvolysis; strained alkynes.
© 2025 The Author(s).ChemBioChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kenny R. G., Marmion C. J., Chem. Rev. 2019, 119, 1058. - PubMed
-
- Annunziata A., Cucciolito M. E., Esposito R., Imbimbo P., Petruk G., Ferraro G., Pinto V., Tuzi A., Monti D. M., Merlino A., Ruffo F., Dalton Trans. 2019, 48, 7794. - PubMed
-
- Annunziata A., Cucciolito M. E., Esposito R., Ferraro G., Monti D. M., Merlino A., Ruffo F., Eur. J. Inorg. Chem. 2020, 918.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

