Enhanced Influenza Vaccines Extend A(H3N2) Antibody Reactivity in Older Adults but Prior Vaccination Effects Persist
- PMID: 40178253
- PMCID: PMC12596410
- DOI: 10.1093/cid/ciaf060
Enhanced Influenza Vaccines Extend A(H3N2) Antibody Reactivity in Older Adults but Prior Vaccination Effects Persist
Abstract
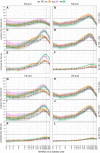
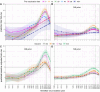
Background: Influenza vaccine effectiveness can be reduced in older adults and among repeatedly vaccinated groups. Results from year 1 of "PIVOT," a randomized trial among adults aged ≥65 years in Hong Kong, showed that adjuvanted (Adj), high-dose (HD), and recombinant hemagglutinin (rHA) vaccines induced greater antibody responses against vaccine viruses than standard-dose (SD) influenza vaccine. Here, we examine the breadth of A(H3N2)-reactive antibodies induced during the first 2 study years (2017/2018, 2018/2019), and compare participants who received influenza vaccination annually, or not at all, for 5 years preceding enrollment.
Methods: 14-20 PIVOT participants per vaccine and prior vaccination group (0/5 or 5/5 prior years) who provided sera on days 0, 30, and 182 in year 1 and days 0 and 30 in year 2 were assessed. Hemagglutination inhibition (HAI) antibody titers were measured against 30 viruses spanning 1968 to 2018.
Results: In year 1, rHA and Adj but not HD vaccines induced titers ≥40 and titer rises ≥4-fold (seroconversion) against significantly more strains than SD vaccine among participants vaccinated 0/5 prior years. Only rHA and Adj vaccines induced titers ≥40 against post-vaccine strains. Antibody responses were poor among participants vaccinated 5/5 compared with 0/5 prior years and only rHA increased the breadth of seroconversion compared with the SD vaccine in this group. Antibody responses were weaker across groups in year 2.
Conclusions: The results suggest that Adj and particularly rHA vaccines may improve the breadth of protection against A(H3N2) viruses but may not overcome attenuating effects of repeated vaccination in older adults.
Clinical trials registration: NCT03330132.
Keywords: immunogenicity; influenza; older adults; public health; vaccination.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. F. reports a relationship with Sanofi Aventis France that includes funding grants and has consulted for Evidera. B. J. C. has consulted for AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur. S. G. S. has consulted for Novavax, CSL Seqirus, Pfizer, Moderna, Evo Health and Astra Zeneca. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Data availability. The data that support the findings of this study are available upon request to the corresponding author.
Figures


References
-
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. - PubMed
-
- Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, I-Cheng MC. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 2017; 35:212–21. - PubMed
-
- Treanor JJ, Schiff GM, Couch RB, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis 2006; 193:1223–8. - PubMed
-
- Baxter R, Patriarca PA, Ensor K, Izikson R, Goldenthal KL, Cox MM. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok(R) trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50–64 years of age. Vaccine 2011; 29:2272–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

