Recombinant Hypoallergenic Cat Allergy Vaccines
- PMID: 40178413
- PMCID: PMC12444911
- DOI: 10.1111/all.16542
Recombinant Hypoallergenic Cat Allergy Vaccines
Abstract
Background: Molecular forms of allergen-specific immunotherapy (AIT) for cat allergy are needed. Fel d 1, Fel d 4, and Fel d 7 are the most important cat allergens.
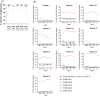
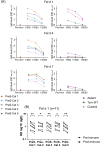
Methods: IgE epitopes of Fel d 4 and Fel d 7 were mapped by blocking allergic patients' IgE binding with allergen peptide-specific antisera. Five recombinant fusion proteins (PreS-Cat 1-PreS-Cat 5) each containing hepatitis B virus (HBV)-derived PreS as an immunological carrier and non-allergenic peptides from the IgE binding sites of Fel d 1, Fel d 4, and Fel d 7 were expressed in Escherichia coli, purified, and characterized by mass spectrometry, circular dichroism (CD), and size exclusion chromatography. ImmunoCAP and basophil activation experiments demonstrated the hypoallergenic activity of PreS-Cat 1-5. The ability of PreS-Cat 1-5 to induce IgE-blocking antibodies in rabbits was compared to three licensed allergen extract-based AIT vaccines. PreS-Cat 1-5-specific IgG antibodies were tested for inhibition of allergen-specific IgE binding and specific basophil activation. T cell activation and induction of specific cytokine secretion by PreS-Cat proteins were compared with cat allergens in PBMC cultures.
Results: Recombinant hypoallergenic, biochemically and structurally defined PreS-Cat 1-5 were obtained. Two subcutaneous immunizations of rabbits with PreS-Cat 1-5 induced equal (Fel d 1) or better (Fel d 4 and Fel d 7) antibodies (PreS-Cat 5 > PreS-Cat 1 > PreS-Cat 3) blocking allergic patients' IgE binding to cat allergens than six to fifteen immunizations with allergen extract-based vaccines. PreS-Cat-specific antibodies strongly inhibited specific basophil activation. PreS-Cat 5 > PreS-Cat 1 induced significantly more IL-10 in cultured PBMCs from cat allergic patients than cat allergens.
Conclusions: PreS-Cat 5 and PreS-Cat 1 are highly promising molecular vaccine candidates for AIT of cat allergy, combining Fel d 1-, Fel d 4-, and Fel d 7-peptides in single PreS fusion proteins.
Keywords: allergen; allergen‐specific immunotherapy (AIT); allergy; cat allergy; molecular allergy vaccine.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Rudolf Valenta has received research grants from WORG Pharmaceutical (Hangzhou, China) and HVD Life Biotech (Vienna, Austria). He serves as a consultant for HVD and WORG Pharmaceuticals. D.T., M.C., K.R., A.K. (Antonina Karsonova), M.v.H., A.K. (Alexander Karaulov) and R.V. are authors of the patent application “Vaccine for treating allergies” application number: PCT/CN2022/130414, reference: P20222370. The authors with a Russian affiliation declare that they have prepared the article in their “personal capacity” and/or that they are employed at an academic/research institution where research or education is the primary function of the entity. M.v.H. has received lecture fees from Astra Zeneca and Thermofisher scientific outside the submitted work. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

